Abstract—The thermal dehydration of GdCl3 ⋅ 6H2O crystalline hydrate is studied by synchronous thermal analysis and mass spectrometry using certified samples. The mechanism and the caloric effects of the chemical reactions are determined. The thermal decomposition of GdCl3 ⋅ 6H2O crystalline hydrate is accompanied by hydrolysis, leading to the formation of oxychloride GdOCl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Rare-earth metals find wide use in various fields of industry, science, and technology. In particular, gadolinium and its compounds are actively used in nuclear power engineering, electronics, and medicine [1]. Among all stable elements, gadolinium is characterized by the highest capability of neutron capturing, is used for protection from thermal neutrons, and, hence, is promising for controlling nuclear reactors. Enamels, ceramics, and paints are prepared on the basis of gadolinium oxide. An alloy of gadolinium with nickel is used for manufacturing containers for burning radioactive wastes. Alloys of gadolinium with cobalt and iron possess unique magnetic properties and, therefore, are applied for manufacturing memory carriers with high recording density. Gadolinium oxides are among the basic components of superconducting ceramics and laser materials. Gadolinium salts are used as contrast substances for medicinal research in magnetic resonance tomography. The main method for the preparation of gadolinium is metallothermy, during which gadolinium is reduced in GdCl3 and GdF3 melts using calcium [1].
It is known that gadolinium chloride (as other rare-earth metal chlorides) is hygroscopic and adsorbs water vapors to form crystalline hydrates, heating of which can result in the formation of an impurity of oxychlorides [2, 3].
This can change the physicochemical properties of an electrolyte and exert a negative effect on the high-temperature chemical processes that occur and on the yield of the target components [1].
The available data [2–7] obtained during recent fifty years on the scheme of the thermal dehydration of gadolinium chloride crystalline hydrates, on the heats of phase transitions, and on the compositions of the intermediate and final phases are contradictory. The thermal decomposition of gadolinium chloride in air was studied by thermogravimetry (TG) [2]. The results showed [2] that the thermal decomposition of GdCl3 ⋅ 6H2O started at 90°C. A decrease in the sample weight on heating to 210°C was 27.1%, which is consistent with the assumption about the formation of the GdCl3 ⋅ H2O crystalline hydrate phase. An anhydrous salt with an impurity of GdOCl oxychloride is formed when temperature increases to 350°C, and the subsequent weight decrease is attributed to the formation of the GdOCl phase.
The thermal dissociation of GdCl3 ⋅ 6H2O in an inert atmosphere (N2) was studied [4] by differential scanning calorimetry (DSC), and the enthalpies of the occurred processes were determined. The following scheme of thermal dehydration is presented on the basis of gravimetric measurements: GdCl3 ⋅ 6H2O → GdCl3 ⋅ 3H2O → GdCl3 ⋅ 2H2O → GdCl3 ⋅ H2O → GdCl3. In this process, the final product (anhydrous GdCl3) is formed at 260°C. The enthalpy of formation of gadolinium chloride trihydrate was 180.5 kJ/mol, but the tensimetric measurements [5] gave the heat of the same reaction of 126.3 kJ/mol.
The data on GdCl3 ⋅ 6H2O dehydration were obtained using a unique procedure and supplemented by chemical analysis results for the final product [6]. The following scheme of thermal dehydration is presented on the basis of the obtained experimental data: GdCl3 ⋅ 6H2O → GdCl3 ⋅ 3H2O → GdCl3 ⋅ 2H2O → GdCl3 ⋅ H2O.
The results of synchronous thermal analysis (STA) including parallel measurements of the sample weight on heating and accompanying thermal effects of gadolinium trichloride crystalline hydrate are presented in one of the recent studies in this field [7]. To prevent hydrolysis, a sample was heated in argon to 230°C. The conclusions made on the basis of the obtained data differed substantially from the earlier conclusions [2–6], and the authors of [7] proposed the following scheme of thermal dehydration: GdCl3 ⋅ 7H2O → GdCl3 ⋅ 4.4H2O → GdCl3 ⋅ 2.4H2O → GdCl3 ⋅ 0.3H2O. It should be mentioned that the temperatures of the marked thermogravimetric and caloric effects do not agree with those in the dehydration scheme.
In our opinion, substantial drawbacks of all studies considered are lacking data on the certification of the initial samples, limited data obtained by thermal analysis methods, and the absence of a combined approach to the investigation of the dehydration process using additional methods of research to establish the type of chemical reactions and the accompanying caloric effects.
The purpose of this work is to obtain data on the mechanism and thermal effects of the dehydration of GdCl3 ⋅ 6H2O crystalline hydrate using STA, mass spectrometry, X-ray diffraction (XRD) analysis, Raman spectroscopy, IR spectroscopy, and calculations of the thermodynamic parameters of the chemical reactions.
EXPERIMENTAL
Experimental Techniques
The temperatures of phase transitions and the accompanying thermal effects were measured and mass spectrometric measurements of the evolved gases were conducted on an STA 449C Jupiter synchronous thermal analyzer equipped with an Aëolos quadrupole mass spectrometer (NETZSCH, Germany). The instrument was calibrated using reference samples of RbNO3, KClO4, Ag2SO4, CsCl, K2CrO4, and biphenyl C10H12 (NETZSCH). The initial sample weight was 10–13 mg, the heating rate was 5°C/min, and the temperature range was 25–500°C. The measurements were carried out in an atmosphere of high-purity (99.998%) argon, and a Pt–Rh alloy crucible served as a container for a sample. According to the technical characteristics of the device, the error of determining the temperature was less than 1°C and the error of measuring the mass was 10–6 g. The experimental data were processed with the licensed Proteus software package.
The synthesized samples of GdCl3 ⋅ 6H2O crystalline hydrate and final products of its thermal decomposition were certified using XRD analysis, Raman spectroscopy, and IR spectroscopy. The phase compositions of the samples were determined on a Rigaku D/MAX-2200VL/PC automated X-ray diffractometer (Japan), a TENSOR 27 FT-IR spectrometer (Bruker, Germany), and an Ava-Raman fiber-optic spectrometric complex (Avantes, Netherlands).
The thermodynamic parameters of the possible chemical reactions in the temperature range 200–500°C were calculated using the HSC 7 Chemistry 8 program package.
Synthesis and Certification of GdCl3 ⋅ 6H2O Crystalline Hydrate
Crystalline hydrate GdCl3 ⋅ 6H2O was synthesized by the reaction
Oxide Gd2O3 (TU 48-4-524-90v) was transferred into a glassy carbon cup and dissolved in an excess of concentrated HCl (reagent grade).
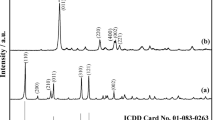
Gadolinium trichloride crystalline hydrate GdCl3 ⋅ 6H2O was prepared by the evaporation of the solution on slow heating to the temperatures not higher than 100°C. XRD analysis of the obtained sample showed the formation of crystalline hydrate GdCl3 ⋅ 6H2O (Fig. 1). The IR and Raman spectra of the synthesized crystalline hydrate contain vibrational bands of medium intensity in a range of 200–400 cm–1 assigned to the Gd–Cl bond, intense vibrational bands attributed to bending symmetric and stretching antisymmetric vibrations of crystallization water molecules in the ranges 1620–1650 and 3100–3500 cm–1, and low-intensity bands in a range of 500–700 cm–1 characteristic of libration vibrations of the metal–OH2 bond (Fig. 2) [8]. The results of XRD certification of the synthesized crystalline hydrate GdCl3 ⋅ 6H2O confirmed its single-phase character, and the IR and Raman spectroscopy data showed the absence of an impurity of adsorbed water, the presence of which in a sample can result in additional caloric and thermogravimetric effects during STA.
RESULTS AND DISCUSSION
The data of STA and mass spectrometry of the evolved gases obtained by heating crystalline hydrate GdCl3 ⋅ 6H2O in a temperature range of 25–500°C are presented in Fig. 3. The TG curve in a temperature range of 20–200°C exhibits three weak steps of the sample weight decrement accompanied by caloric effects in the DSC curve at 133, 152, and 170°C, and H2O molecules are detected in the gas phase. The TG curve in a temperature range of 200–275°C contains one step of the weight decrement corresponding to the caloric effect in the DSC curve with the peak maximum at 238°C accompanied by the detection of H2O and HCl molecules in the gas phase. The further heating of the sample in a temperature range of 300–375°C results in the detection of a stepwise decrease in the sample weight accompanied by the heat uptake with a maximum of the effect at 358°C, and hydrogen chloride molecules appear in the gas phase. The reproducibility of the TG and DSC signals was confirmed in a series of experiments.
A general analysis of the obtained experimental data enables us to propose the following scheme of the thermal dehydration of crystalline hydrate GdCl3 ⋅ 6H2O: stepwise separation of water molecules occurs in a temperature range of 25–200°C according to the reactions
The change in the sample weight calculated by Eqs. (2)–(4) is consistent with the weight change detected experimentally (25.9%).
Hydrolysis occurs upon further heating of the sample in the temperature range 200–275°C along with anhydrous salt formation. The hydrolysis affords gadolinium oxychloride according to the following reactions:
The heating of anhydrous gadolinium chloride in the closed space of the working chamber of the instrument in the presence of water vapors leads to its hydrolysis at temperatures of 300–375°C via the reaction
Thus, the overall equation of the thermal dehydration of gadolinium chloride crystalline hydrate can be written as follows:
This conclusion is confirmed by a comparison of the results of the sample weight change (turned out to be 43.8%) calculated by Eq. (8) and the data of thermogravimetric measurements in a temperature range of 25–500°C (weight decrease 38.3%). It should be mentioned that the experimentally observed trend of a sample weight decrease is not finished at 500°C (maximum experimental temperature).
The XRD data for the reaction product confirmed the formation of the GdOCl phase (Fig. 4).
The IR and Raman spectra of the final product exhibit vibrational bands at 535 and 400 and 182, 235, 383, and 508 cm–1, respectively, which is consistent with the data for the vibrational spectra of GdOCl [9, 10].
The peaks of the DSC curve were separated and the thermal effects accompanying the occurred chemical reactions were estimated by the results of caloric measurements of the dehydration of crystalline hydrate GdCl3 ⋅ 6H2O using the PEAK SEPARATION software. The data obtained are presented in Fig. 5 and Table 1.
The thermodynamic parameters of reaction (8) were additionally calculated using the HSC 7 Chemistry 8 program package, and the results are presented in Table 2.
It turned out that the specific amount of heat absorbed by the system during this reaction was about 474 kJ/mol at 500°C (Table 2), which is comparable with the total thermal effect of reactions (2)–(4) (423 kJ/mol) determined by DSC measurements. Interestingly, according to the calculation results (Table 2), the hydrolysis of crystalline hydrate GdCl3 ⋅ 6H2O begins in a temperature range of 200–250°C. This finding agrees with the experimental data obtained by the investigation of the dehydration process using a mass spectrometric study of the evolved gases (Fig. 3).
CONCLUSIONS
(1) The studies of the thermal dehydration of crystalline hydrate GdCl3 ⋅ 6H2O were carried out by STA and mass spectrometry using certified samples.
(2) The phase composition changes on heating crystalline hydrate GdCl3 ⋅ 6H2O according to the following scheme: GdCl3 ⋅ 6H2O → GdCl3 ⋅ 3H2O → GdCl3 ⋅ 2H2O → GdCl3 ⋅ H2O → GdCl3 + GdOCl → GdOCl. The final thermal decomposition product was certified by XRD analysis, Raman spectroscopy, and IR spectroscopy.
(3) Hydrolysis occurs along with anhydrous salt formation in a temperature range of 200–275°C to form gadolinium oxychloride.
(4) The peaks in the DSC curve were separated and the thermal effects accompanying the occurred chemical reactions were estimated using the PEAK SEPARATION software.
REFERENCES
C. K. Gupta and N. Krishnamurthy, Extractive Metallurgy of Rare Earths (CRC PRESS, London, 2005).
W. Wendlandt, “The thermal decomposition of yttrium, scandium, and some rare-earth chloride hydrates,” J. Inorg. Nucl. Chem. 5, 118–122 (1957).
W. Wendlandt, “The thermal decomposition of the heavier rare earth metal chloride hydrates,” J. Inorg. Nucl. Chem. 5, 136–139 (1959).
S. Ashcroft and C. Mortimer, “The thermal decomposition of lanthanide(III) chloride hydrates,” J. Less-Common Metals 14, 403–406 (1968).
N. P. Sokolova and E. A. Ukraintsev, “Pressure of dissociation of some crystalline hydrates of lanthanide chlorides,” Izv. Sib. Otd. Akad. Nauk SSSR, Ser. Khim., No. 1, 24–25 (1984).
V. V. Hong and J. Sundstrum, “The dehydration schemes of rare-earth chlorides,” Thermochim. Acta 307, 37–43 (1997).
Tae-Kyo Lee, Yong-Zun Cho, Hee-Chul Eun, Sung-Mo Som, In-Tae Kim, and Taek-Sung Hwang, “A study on dehydration of rare earth chloride hydrate,” J. Korean Radioactive Waste Soc. 10, 125–132 (2012).
V. N. Makatun, Chemistry of Inorganic Hydrates (Nauka i Tekhnika, Moscow, 1985).
Y. Hase, P. Dunstan, and M. Temperini, “Raman active normal vibrations of lanthanide oxychlorides,” Spectrochim. Acta A: Mol. Spectrosc. 37 597–599 (1981).
L. Basile, J. Ferraro, and D. I. R. Gronert, “Spectra of several lanthanide oxyhalides,” J. Inorg. Nucl. Chem. 33, 1053–1074 (1971).
ACKNOWLEDGMENTS
The authors are grateful to B.D. Antonov (Center for Collective Use Composition of Matter, Institute of High-Temperature Electrochemistry, Ural Branch, Russian Academy of Sciences) for performing the XRD analysis.
This work was supported in part by the Russian Foundation for Basic Research, project no. 18-03-00561a.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Korzun, I.V., Zakir’yanova, I.D. & Nikolaeva, E.V. Mechanism and Caloric Effects of the Thermal Dehydration of GdCl3 ⋅ 6H2O Crystalline Hydrate. Russ. Metall. 2018, 722–727 (2018). https://doi.org/10.1134/S0036029518080104
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029518080104