Abstract
In this work, the electrical conductance approach is used to investigate the molecular interactions between nonessential amino acid (L-glutamic acid) and carbohydrates (L-arabinose/D-xylose) at various temperatures. Many characteristics, including limiting molar conductance (\({{\Lambda }}_{{\text{m}}}^{0}\)), Walden product (\({{\Lambda }}_{{\text{m}}}^{0}{{\eta }_{0}}\)), activation energy (\({{E}_{{\text{A}}}}\)), and thermodynamic parameters, were obtained for the binary and ternary solutions of L-glutamic acid (Glu) from the electrical conductance (\(\hat {k}\)) measurement. The acquired thermodynamic quantities were interpreted in terms of the systems’ physicochemical interactions. The behavior of small biomolecules (amino acid) and saccharides in solute-solvent interactions is shown by the influence of numerous parameters, including concentration, temperature, and the position of axial and equatorial –OH groups of the saccharides, on these quantities. Hydrophilic-hydrophilic or ion-hydrophilic interactions predominate in the systems that are being studied is supported by the UV–Vis spectroscopy investigations’ absorption values.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 INTRODUCTION
All of the biochemical activities that take place in living things depend on proteins, which are the fundamental biomolecules. Protein’s structural characteristics are significantly influenced by how they interact with their surroundings. Understanding these interactions is crucial for understanding the unfolding behavior and conformational stability of globular proteins. Direct thermodynamic research of these biomolecules is rather challenging due to their structural complexity. Since amino acids are the building blocks of proteins, they are frequently employed as model molecules for understanding the interaction of protein with other system [1–4]. The stabilization of globular proteins in their original state and the lowering of the degree of denaturation by other substances are enhanced by polyhydroxy compounds, including carbohydrates. It has been observed that the number of hydroxyl groups in carbohydrates affects their stability [5, 6]. The interactions between proteins and carbohydrates are essential for a variety of biochemical processes in living systems, including immunology, biosynthesis, pharmacology, medicine, and the cosmetics industry. Carbohydrates found at cell surfaces are significant as receptors for the bioactive structures of enzymes, hormones, viruses, antibodies, etc. [7–9]. Several experimental paper reports physicochemical properties of certain amino acids in aqueous carbohydrate solutions [10–14]. In our recent papers we report the density, viscosity, spectroscopic characteristics and taste behavior of L-glutamic acid and L-aspartic acid in carbohydrates solution [15, 16]. Many parameters, such as temperature, viscosity, the kind of solvent/solvent interaction, hydrogen bonding, ion solvation, etc., typically have an impact on the conductivity of a solute in a solvent. Conductometric study play a key role in investigation of molecular interaction but there are not sufficient data on conductivity study of amino acids.
Considering this, the present work has been made to study the molecular interaction using conductivity and UV–Vis spectrophotometric techniques. In this study, molar conductivities of amino acids (L-glutamic acid) at temperature ranges of 293.15 to 313.15 K have been determined in aqueous solutions of D‑xylose/L-arabinose (2.0, 2.5, and 3.0 wt %). Several parameters, including molar conductance (\({{{{\Lambda }}}_{{\text{m}}}}\)), limiting molar conductance (\({{\Lambda }}_{{\text{m}}}^{0}\)), change in enthalpy (ΔH), entropy (ΔS), free energy (ΔG), and activation energy (\({{E}_{{\text{A}}}}\)) of the system can be determined from the electrical conductance data. The absorbance of Glu was studied in the absence and presence of L-arabinose/D-xylose and were compared with the findings obtained from our recently published article. The solute-solute and solute-solvent interactions in these systems have been discussed using these parameters.
2 EXPEIMENTAL
2.1 Materials
Preparation of solutions. Monosaccharides L‑arabinose and D-xylose, as well as the amino acid L-glutamic acid, were purchased and used as standard without further purification; however, to prevent moisture absorption, they were dried over P2O5 for 48 h in a desiccator. Table 1 provides a comprehensive description of the compounds used in the current study. Deionized water was mixed with a specified quantity of Glu to made aqueous glu solutions. L‑arabinose/D-xylose solutions (2.0, 2.5, and 3 wt %) were also made using the deionized water. Throughout the whole experiment, aqueous solutions of 2.0, 2.5, and 3 wt % L-arabinose/D-xylose and water were used as solvents to create molal solutions of Glu (0.01, 0.02, 0.03, 0.04, and 0.05 mol kg–1). At room temperature (T = 298.15 K) and pressure (p = 101 kPa), all of the solutions were prepared. The uncertainty of molality of the solutions was determined to be ±1 × 10–3 mol kg–1.
2.2 Measurement of Conductance
A digital conductivity meter (model 306) by Systronics having cell constant 1.0 cm–1 (±10%) was employed to measure specific conductance of the solutions at T = 293.15, 298.15, 303.15, 308.15, and 313.15 K. Each solution was kept in a thermostatically controlled water bath for 10 min to attend the experimental temperatures [17]. A glass electrode of type \({\text{CD}}10\) was dipped in the solution and specific conductance reading (\(\hat {k}\)) displayed on a LED screen was noted. Average of 3 to 4 readings taken at an interval of 10 s was considered. The instrument was calibrated with 0.01M\({\text{ KCl}}\). Accuracy of specific conductance is ±0.1%.
2.3 UV-Visible Absorption Spectroscopy
When comparing a sample to a reference or blank sample, the analytical technique called UV–Vis spectroscopy counts the number of discrete wavelengths of UV or visible light that are absorbed by or transmitted through the sample. In the present investigation, the absorption spectra of Glu in water and aqueous L‑arabinose/D-xylose were recorded using a UV-visible spectrophotometer (SYSTRONICS DOUBLE BEAM UV–VIS Spectrophotometer: 2202), where a quartz cuvette with a 1 cm route length was employed. The wavelength range was 200–400 nm. Baseline adjustments were performed to each absorption spectra that was recorded. The absorption spectra of every sample were recorded thrice, and the interpretation of \({{\lambda }_{{{\text{max}}}}}\) was based on the average absorption value.
3 RESULTS AND DISCUSSION
3.1 Conductance Studies
3.1.1. Molar conductance and limiting molar conductance. The conductance feature of a homogeneous solution containing one mole of electrolyte is ascertained by the molar conductivity, \({{{{\Lambda }}}_{{\text{m}}}}\) (pivotal in the movement of ions) which helps to discriminate the ionic strength of a solution. During the investigation of extensive applications of amino acids in industrial as well as pharmaceutical developments conductivity plays a vital role. From the obtained specific conductivity (\(\hat {k}\)) results, which are listed in Table 2 molar conductivity, \({{{{\Lambda }}}_{{\text{m}}}}\) was evaluated with the help of Eq. (1)
where c is Concentration of solution in molarity.
Molality (m, mol kg–1) was converted to molarity (c, mol m–3) with the help of an usual equation,
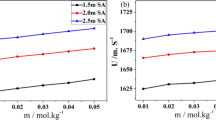
Molecular mass of Glu, and molality and density of Glu solutions are denoted by \({{M}_{{\text{A}}}}\), \({{m}_{{\text{A}}}}\), and \({{\rho }}\), respectively. The variation of specific conductance for L‑glutamic acid of different concentrations (\({{m}_{{\text{A}}}}\)) in aqueous and aqueous L-arabinose/D-xylose at different temperatures are shown in Fig. 1.
Another important characteristic trait of electrolytic solutions is conductance that governs the extent of dissociation, as well as the type of solvent-solvent, solute-solvent and solute-solute interactions. The study on conductometric properties furnish kinetic information approaching ionic conductivities and thermodynamic knowledge approaching association constants.
Electrical conductivity of Glu solutions in 2.0, 2.5, and 3.0 wt % aqueous L-arabinose/D-xylose was measured at T = 293.15–313.15 K. Experimentally measured specific conductance (\(\hat {k}\)) was converted to molar conductance \(({{{{\Lambda }}}_{{\text{m}}}})\) according to Eqs. (2)–(4).
By least squares fitting of \({{{{\Lambda }}}_{{\text{m}}}}\) data, the limiting molar conductivity, \({{\Lambda }}_{{\text{m}}}^{0}\) of solutions of Glu was obtained by Eq. (4) (Ostwald’s dilution law) [18]
when \({{{{\Lambda }}}_{{\text{m}}}}\) is plotted against \({{c}^{{1/2}}}\) (the linear regression), the intercept gives \({{\Lambda }}_{{\text{m}}}^{0}\) value and slope gives S values.
The change in \({{{{\Lambda }}}_{{\text{m}}}}\) with \({{c}^{{1/2}}},\) (square root of molar concentration) of the Glu solutions is graphically shown in Fig. 2 and the intercept \(({{\Lambda }}_{{\text{m}}}^{0},\) limiting molar conductivity) data of the plots are listed in Table 3.
The potent intercommunications among the axial and equatorial –OH ions of L-arabinose /D-xylose and zwitterionic groups of Glu influence the degree of dissociation of the solute and so also the molar conductivity since molar conductivity is the conducting ability of all the ions when one mole of an electrolyte gets completely dissolved in solution. As can be observed from Fig. 2 that the values of \({{{{\Lambda }}}_{{\text{m}}}}\) decrease with a rise in concentration of Glu solutions in water and in all the compositions of L-arabinose/D-xylose at each experimental temperature. With increase in content of solute ions in water more molecules of water gets engaged in hydration process. As the size of hydrated ions are larger their mobility gets reduced decreasing the conductivity. Carbohydrates used in the present work dissociate completely in water as they are strong electrolytes and generate more ions. But it is found that there is a rise in electrical conductivity of Glu solutions in aqueous L-arabinose/D-xylose system which may be due to ion-ion/ion-solvent, or solute-cosolute interactions. Furthermore, with a rise in composition of cosolute (L-arabinose/D-xylose) ion association turns up with the evolution of contact ion pairs, solvent separated and/or solvent shared moieties leading to decrease in mobility [19].
On the other hand, in spite of evolution of large ions of less mobility the electrical conductivity gets increased considerably because of the existence of more number of ions in the solution in ternary phase. With a rise in temperature thermal expansion of migrating ions propagate its translational, rotational and vibrational energy leading to rapid transfer of electric charge resulting a rise in conductance.
Estimation of limiting molar conductance \(({{\Lambda }}_{{\text{m}}}^{0})\) furnishes satisfactory results on mainly ion-solvent interactions. Because at infinite dilution the ions are at infinite distance and the mobility of the ions are mostly regulated by the association of those ions with that of water molecules surrounding them. Hence, ion – pair formation is hindered at this situation and electrical charge transfer and molar conductance gets enhanced. The eye on Table 3 and Fig. 3 indicates that \({{\Lambda }}_{{\text{m}}}^{0}\) increased as the temperature rises for all compositions of L-arabinose/D-xylose showing lower solvation ensuing greater ionic mobility. Viscosity plays a dominating character in solution media with respect to mobility of ions. The increase in temperature decreases the viscosity of the medium because of the greater randomness of ions which is the result of cleavage of hydrogen bonds and ion-hydrophilic bonds among the molecules of solute-cosolute as well as water. As the solution is at lower viscous state the mobility of ions are higher and hence electrical conductivity is more [20].
3.1.2. Walden product. As observed the electrical conductivity increases upon a reduction in viscosity. Paul Walden (1960) remarked that the product of \({{\Lambda }}_{{\text{m}}}^{0}\) and \({{{{\eta }}}_{0}}\), which are the molar conductivity and viscosity at infinite dilution of a solution is a constant called Walden product (\({{\Lambda }}_{{\text{m}}}^{0}{{{{\eta }}}_{0}}\)). It is used in order to examine the studied solute acts as a structure breaker or structure maker for the chosen solvent systems. Or we can say Walden product is helpful in anticipating the capability of a solute in a solution to rebuild the structure of water. For the present investigation the values of Walden products are given in Table 3. It is monitored from Table 3 Walden products for Glu show a rising trend in aqueous medium and a downward trend in aqueous L-arabinose/D-xylose as the temperature gets increased. Positive (\({{\Lambda }}_{{\text{m}}}^{0}{{{{\eta }}}_{0}}\)) values support the amino acid’s structure-building behavior when an electrolyte is present [15].
Since temperature shows a positive impact on conductance and opposite impact on viscosity, these two properties are reciprocal to one another. So the trend of Walden product does not following a formal variation with temperature. When cosolutes (L-arabinose/D-xylose) are added the solvation of ions gets reshaped. Some molecules of water gets substituted by the ions of electrolyte or cosolute. The two opposing parameters \({{\Lambda }}_{{\text{m}}}^{0}\) and \({{{{\eta }}}_{0}}\) results to a decreasing variation of Walden products.
3.1.3. Activation energy. In the solution the charged particles drift from lower to higher energy level. This higher energy level with less stability is known as activated complex or transition state. Thus, for the transition of prevailing ions or molecules from one electrode to other an effective potential energy barrier must be apprehended. That energy which is required to attain this transition state is given by \({{E}_{{\text{A}}}}\), called activation energy of charge transfer. The Arrhenius equation can be adapted to depict the effect of temperature upon \(\Lambda _{{\text{m}}}^{0}\) as shown in Eq. (5). Using this equation \({{E}_{{{\text{A }}}}}\) values can be evaluated, if the values of ln\(\Lambda _{{\text{m}}}^{0}\) is plotted against \(1{\text{/}}T\), values of \({{E}_{{{\text{A }}}}}\) can be collected from the slope of the line. For the present study the variation is shown in Fig. 4 and the \({{E}_{{{\text{A }}}}}\) values are given in Table 3. The higher \({{R}^{2}}\) values (Fig. 4) recommended the limiting molar conductance convention of temperature well fitted with Arrhenius equation [21]
where A, R and T are Arrhenius constant, ideal gas constant, and absolute temperature, respectively. As \({{E}_{{{\text{A }}}}}\) values are obtained positive it can be said that the solubilization of Glu is in favor of the presence of cosolute (L‑arabinose/D-xylose) and hence reveal the existence of ion-solvent interactions which are in well agreement with the volumetric and viscometric results.
3.1.4. Thermodynamics of ion association. The nature of contacts, especially those involving electrostatic, hydrogen bonding, and hydrophobic interactions, can be partially explained by the Debye–Hückel theory and its expansions. It is crucial to investigate and ascertain the thermodynamic characteristics associated with ion association and ion pair formation since they are crucial to the movement of charged particles in the medium [15, 16]. The structural alterations of solvent molecules surrounding the cations and anions seem to regulate the thermodynamic properties including heat of dilution, entropy changes, and activity coefficient fluctuations under different conditions of temperature, concentration, pH, etc. The following set of equations can be used to quantify changes in conventional Gibb’s thermodynamic parameters, such as free energy (\(\Delta G_{{\text{A}}}^{0}\)), entropy (\(\Delta S_{{\text{A}}}^{0}{{\;} }\)), and enthalpy (\(\Delta H_{{\text{A}}}^{0}\)), that are involved in the ion association rate process [21].
Equilibrium constant for the association process (\({{K}_{{\text{A}}}}\)) and standard free energy change can be connected as shown in the following equation,
The way to express \({{K}_{{\text{A}}}}\) is as,
Equation (8), when applied, yields the degree of dissociation (α)
The deduced values of \({{K}_{{\text{A}}}}\) are given in Table 4. The following is how the Gibbs–Helmholtz equation, which is crucial for figuring out the relationships between the thermodynamic parameters, is stated,
Equation (10), which is derived from the Gibbs–Helmholtz equation, further connects the temperature dependence of the change in Gibbs energy with the system’s enthalpy
It can also be written as,
Equation (10) states that if \(\Delta G_{{\text{A}}}^{0}\) is plotted against temperature T, as shown in Fig. 5, the slope of the straight line yields the value of the rate at which \(\Delta G_{{\text{A}}}^{0}\) changes with temperature. Equation (11), on the other hand, gives the value of \( - \Delta S_{{\text{A}}}^{0}\). The value of \(\Delta H_{{\text{A}}}^{0}\) is obtained from the straight line’s intercept.
Table 5 provides values for the thermodynamic parameters described previously. It is discovered that \(\Delta G_{{\text{A}}}^{0}\) for Glu in aqueous solutions is larger than zero. It suggests that the production of ions in pairs is not possible thermodynamically in the absence of electrolytes. Ion association becomes spontaneous and possible (negative \(\Delta G_{{\text{A}}}^{0}\)) as the amount of co-solute (L‑arabinose/D-xylose) in the solutions increases. With rising temperatures, they become more negative. The growing negative value with temperature is explained by two causes, namely the growth in \(\Delta H_{{\text{A}}}^{0}\) and \(\Delta S_{{\text{A}}}^{0}\) values.
It is found that ion association is exothermic thermodynamically for all Glu in water and aqueous 2.0 wt % L-arabinose. The reason for the negative values of \(\Delta H_{{\text{A}}}^{0}\) could be the electrostatic contact between the anionic sites and cationic head groups of Glu and L-arabinose/D-xylose. It’s crucial to remember that the value of \(\Delta H_{{\text{A}}}^{0}\) isn’t always negative; on occasion, it could even be positive based on the surfactant’s structural characteristics, the environment of the solution, the presence of additional additives and cosolvents, etc. As an instance, the positive values of H for sodium N-dodecanoyl sarcosinate in an aqueous medium at 293 and 298 K, respectively, are 42.27 and 12.24 kJ mol–1 [22]. Hence, in the present study except in water and aqueous 2.0 wt % L-arabinose the values of \(\Delta H_{{\text{A}}}^{0}\) for Glu are found to be positive in the rest of the solvent compositions of the chosen systems. This suggests, the ion association process is endothermic, meaning that a significant amount of energy is needed to start the creation of ion pairs. The values of \(\Delta H_{{\text{A}}}^{0}\) climb with temperature. Positive \(\Delta H_{{\text{A}}}^{0}\) values arise from the hydrophobic interactions between the hydrocarbon backbone of L-arabinose/D-xylose and the alkyl segment of Glu. However, hydration of the amino acid polar heads and polar L-arabinose/D-xylose sites have a detrimental effect on \(\Delta H_{{\text{A}}}^{0}\) [23, 24]. Dehydration of the hydration shells occurs in the presence of cosolute in the solution as a result of interactions between the zwitterionic centers of Glu and L‑arabinose/D-xylose. When water molecules are released, there are more degrees of freedom upsetting the surrounding organized water molecules which results in positive \(\Delta S_{{\text{A}}}^{0}\). However, this makes the system more entropic overall [25–28].
The estimate of the change in heat capacity \((\Delta C_{{(p,~m)}}^{0} = {{(\partial \Delta H_{m}^{0}{\text{/}}\partial T)}_{p}})\) has been made using the slope from the plot of \(\Delta H_{{\text{A}}}^{0}\) vs. T in Fig. 6. The values of \(\Delta C_{{(p,~m)}}^{0}\) for Glu in aqueous L-arabinose/D-xylose are found to be positive (Table 5). Although the hydrophilic head groups remained hydrated, it may be hypothesized that the primary reason of the change in heat capacity is the differences in contact of the water molecules with the hydrophobic tails during association [29, 30].
The interaction between zwitterionic centers of amino acids and polar groups of L-arabinose/D-xylose involves a combination of electrostatic forces and hydrogen bonding. The specific amino acids and their spatial arrangement contribute to the strength and specificity of these interactions. The understanding of such molecular interactions is crucial in fields like biochemistry and molecular biology, where the study of protein-carbohydrate interactions is relevant. Glutamic acid is an amino acid that can exist in different protonation states and L-arabinose/D-xylose can exist in different conformations. Changes in temperature or interactions with other components might induce conformational changes in glutamic acid and L-arabinose/D-xylose influencing the heat capacity. Changes in the disorder or randomness of the system, such as the mixing of different solutes in a solution, can contribute to changes in entropy. The entropy change, along with enthalpy changes, influences the overall change in heat capacity. It’s important to note that the positive change in heat capacity does not necessarily point to a single factor but is a cumulative effect of various thermodynamic processes occurring in the solution. Experimental measurements, such as calorimetry, can help quantify these changes and provide a more detailed understanding of the specific contributions to the positive change in heat capacity in your ternary solution.
3.2 UV Spectral Analysis
Glutamic acid contains functional groups like amino (–NH2) and carboxyl (–COOH) groups. In UV–Vis spectroscopy, these groups can contribute to the absorption spectrum. The exact absorption peaks will depend on the pH of the solution because the amino and carboxyl groups can exist in different ionization states [15, 16]. Carbohydrates like xylose and arabinose are generally not strongly absorbing in the UV–Vis range. However, some absorption might be observed due to the presence of conjugated double bonds or other chromophoric groups [31]. When performing UV–Vis spectroscopy on a solution containing a mixture of Glu, L-arabinose/D-xylose a combined spectrum is obtained. The absorption peaks will be a sum of the individual contributions from each component. The absorption spectra of Glu in water, as well as aqueous L-arabinose and D-xylose, were recorded in the current investigation using a UV-visible spectrophotometer. The absorbance vs. wavelength graphs for Glu (0.03 M) in water, aqueous L-arabinose, and aqueous D-xylose (2.0 wt %) are displayed in Fig. 7. Because the compounds taken contain C=O, –NH2, and –OH groups, the absorption spectra of the current system are characterized by Π-Π* and n-Π* electronic transitions. As can be seen, Glu exhibits a wide absorption band in the 200–250 nm range in its UV spectra. In the absence of L-arabinose/D-xylose, the absorption spectra of aqueous Glu (0.03 mol kg–1) show a \({{\lambda }_{{{\text{max}}}}}\) value of 214.4 nm. The Π-Π* absorption band at 215.6 nm in aqueous 2.0 wt % L-arabinose has been shown to have a bathochromic shift in Glu, whereas at 215.4 nm in aqueous 2.0 wt % D-xylose. The hydrophilic-hydrophilic and ion-hydrophilic interactions between Glu and aqueous saccharides are responsible for the bathochromic shift of the Π-Π* absorption band in aqueous L-arabinose and D-xylose. L-arabinose and D-xylose have 1e2e3e4a and 1e2e3e4e conformations, respectively [16]. D-xylose better fits into water molecules than L-arabinose because of spatial arrangement in their –OH groups.
This transition demonstrates that Glu’s strong ion-hydrophilic/hydrophilic-hydrophilic interactions with aqueous saccharides stabilize the excited state more than the ground state. Consequently, the ground-excited state energy differential diminishes, and the band shifts (214.4–215.6 and 215.4 nm) in the direction of the longer wavelength. It may be deduced from the \({{\lambda }_{{{\text{max}}}}}\) values that Glu interacts with L-arabinose more actively than it does with water or aqueous D-xylose. The similar results were obtained for Asp in water and aqueous L-arabinose/D-xylose, where Asp interaction with L-arabinose was more that its interaction with D-xylose [16]. To get more useful and understandable results, experimental optimization is required, which includes modifying pH, concentration, etc.
4 CONCLUSIONS
The impact of carbohyrates, L-arabinose/D-xylose on the conductometric characteristics of the amino acid, Glu at T = 293.15–313.15 K has been demonstrated. The ion association process is shown by the molar conductivity (\({{{{\Lambda }}}_{{\text{m}}}}\)), which is a measure of a solution’s conductivity. Ion mobility is hampered when large ion pairs form. Ion mobility is improved by higher translational and thermal energy. The behavior of amino acids to construct structures in the presence of an electrolyte is supported by positive values of the Walden product (\({{\Lambda }}_{{\text{m}}}^{0}{{{{\eta }}}_{0}}\)). The process of spontaneous ion association between amino acid zwitter ions and ions of L-arabinose/D-xylose is represented by the negative standard free energy change \((\Delta G_{{\text{A}}}^{0})\). The process of ion association is endothermic, leading to an increase in system entropy. This is because the formation of ion pairs releases water molecules from their hydration shells. The observed effects are thought to be caused by modifications in the hydration sphere around the cations and anions, the presence of ion-ion attractions attenuated by H-bonding with water molecules, and hydrophobic interactions of non-polar moieties constituting the anions. The spectroscopic analysis shows that Glu has a more active ionic interaction with L-arabinose than it does with aqueous D-xylose or water. It can be concluded that the systems under study are dominated by ion-hydrophilic or hydrophilic-hydrophilic interactions.
REFERENCES
T. Banerjee and N. Kishore, J. Solution Chem. 34, 2746 (2005). https://doi.org/10.1007/s10953-005-2746-8
A. Ali, S. Khan, S. Hyder, and M. Tariq, J. Chem. Thermodyn. 39, 613 (2007). https://doi.org/10.1016/j.jct.2006.08.010
Z. Yan, J. Wang, W. Kong, and J. Lu, Fluid Phase Equilib. 215, 143 (2004). https://doi.org/10.1016/j.fluid.2003.07.001
T. Banerjee and N. Kishore, J. Solution Chem. 35, 1389 (2006). https://doi.org/10.1007/s10953-006-9069-2
J. F. Back, D. Oakenfull, and M. B. Smith, Biochemistry 18, 23 (1979). https://doi.org/10.1021/bi00590a025
Y. Fujita, Y. Iwasa, and Y. Noda, Bull. Chem. Soc. Jpn. 55, 6 (1982). https://doi.org/10.1246/bcsj.55.1896
G. A. Kulikova and E. V. Parfenyuk, J. Solution Chem. 37 (2008). https://doi.org/10.1007/s10953-008-9275-1
J. A. Siddique, S. Sharma, U. Rashid, K. E. Geckeler, and S. Naqvi, J. Solution Chem. 39 (2014). https://doi.org/10.1007/s13369-014-1114-7
M. Wusteman, S. Boylan and D. E. Pegg, Cryobiology 33, 4 (1996). https://doi.org/10.1006/cryo.1996.0042
A. Nain, J. Mol. Liq. 318, 114190 (2020). https://doi.org/10.1016/j.molliq.2020.114190
A. K. Nain and M. Lather, Phys. Chem. Liq. 53, 599 (2015). https://doi.org/10.1080/00319104.2015.1018256
A. K. Nain, M. Lather, and Neetu, J. Chem. Thermodyn. 63, 67 (2013). https://doi.org/10.1016/j.jct.2013.04.001
A. K. Nain, Renu Pal, and Neetu, J. Chem. Thermodyn. 68, 77 (2014). https://doi.org/10.1016/j.jct.2015.11.015
T. S. Banipal, D. Kaur, P. K. Banipal, and G. Singh, J. Chem. Thermodyn. 39, 3 (2007). https://doi.org/10.1016/j.jct.2006.08.003
R. K. Pradhan, L. Sahoo, and S. Singh, J. Chem. Thermodyn. 189, 107193 (2024). https://doi.org/10.1016/j.jct.2023.107193
R. K. Pradhan and S. Singh, J. Mol. Liq. 394, 123788 (2024). https://doi.org/10.1016/j.molliq.2023.123788
K. Dhal, S. Singh, and M. Talukdar, J. Mol. Liq. B 368, 120761 (2022). https://doi.org/10.1016/j.molliq.2022.120761
R. C. Thakur, A. Sharma, R. Sharma, and H. Kaur, J. Mol. Liq. 374, 121244 (2023). https://doi.org/10.1016/j.molliq.2023.121244
A. Wahab and S. Mahiuddin, J. Chem. Eng. Data 49, 126 (2004). https://doi.org/10.1021/je0302001
N. F. Zolkiflee, M. M. R. M. M. Affandi, and A. B. A. Majeed, Eur. J. Pharm. Sci. 141, 105111 (2020). https://doi.org/10.1016/j.ejps.2019.105111
K. Dhal, S. Singh, and M. Talukdar, J. Sol. Chem. 52, 1415 (2023). https://doi.org/10.1007/s10953-023-01321-z
G. Ray, S. Ghosh, and S. P. Moulik, Surfact. Deterg. 12, 131 (2009). https://doi.org/10.1007/s11743-008-1105-3
M. D. Hossain and M. A. Hoque, J. Chem. Thermodyn. 69, 12 (2014). https://doi.org/10.1016/j.jct.2013.09.030
C. S. Solanki, P. Mishra, M. K. Talari, and M. N. Tripathy, E-J. Chem. 9, 21 (2012). https://doi.org/10.1155/2012/616943
G. B. Ray, S. Ghosh, and S. P. Moulik, J. Surfact. Deterg. 12, 131 (2009). https://doi.org/10.1007/s11743-008-1105-3
S. Singh, M. Talukdar, and U. N. Dash, Russ. J. Phys. Chem. A 96, S8 (2022). https://doi.org/10.1134/S0036024422140229
L. Sahoo, R. K. Pradhan, M. Mohapatra, and S. Singh, Russ. J. Phys. Chem. A 97, 3276 (2023).
H. Shekaari, A. Kazempour, and M. Khoshalhan, Electrochim. Acta 147, 360 (2014). https://doi.org/10.1016/j.electacta.2014.09.101
J. Rozamliana, J. Gurung, and A. K. Pulikkal, J. Mol. Liq. B 390, 123094 (2023). https://doi.org/10.1016/j.molliq.2023.123094
R. K. Pradhan, M. Talukdar, and S. Singh, Russ. J. Phys. Chem. A 97, 2631 (2023). https://doi.org/10.1134/S0036024423120257
Richu, A. Bandral, H. Singh, and A. Kumar, J. Mol. Liq. 364, 120024 (2022). https://doi.org/10.1016/j.molliq.2022.120024
ACKNOWLEDGMENTS
The authors are extremely appreciative of the research facilities offered by the Department of Chemistry at Siksha O Anusandhan Deemed to be University, Odisha.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Contributions
RKP: Conceptualization, methodology, design, formal analysis and interpretation of the available data, visualization, editing, writing and review. SS: Conceptualization, methodology, validation, writing original draft or revising it critically for important intellectual content, supervision, approval of the final version.
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rupesh Kumar Pradhan, Sulochana Singh Conductometric Properties and UV–Vis Spectroscopic Study of L-Glutamic Acid in Aqueous Solutions of L-Arabinose and D-Xylose in the Temperature Range of 293.15–313.15 K at Atmospheric Pressure. Russ. J. Phys. Chem. 98, 2262–2275 (2024). https://doi.org/10.1134/S0036024424701425
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024424701425