Abstract
The thermal effects of acidic and basic dissociation of glycyl-D-phenylalanine dipeptide at a temperature of 298.15 K and ionic strengths of solution of 0.5, 0.75, and 1.0 M against the background of different supporting electrolytes were calculated from the results of direct calorimetric measurements performed on a calorimeter with an isothermal shell and automatic recording of the temperature–time curve. The influence of the nature of supporting electrolytes NaCl, NaClO4, NaNO3, KNO3, and LiNO3 on the thermal effects of stepwise dissociation of the dipeptide is considered. The standard thermal effects of ionization of glycyl‑D‑phenylalanine in two steps were found by extrapolation to zero ionic strength. The standard changes in thermodynamic functions (enthalpy, entropy, and Gibbs energy) in the acidic and basic dissociation of g-lycyl-D-phenylalanine dipeptide were calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Today numerous pharmaceutical products based on peptides can be used as modern and highly effective drugs. Pharmaceutical compositions for the delivery of nuclear hormones, their derivatives, and non-glucagon hormones can be developed on the basis of peptides and their derivatives. Short dipeptides can be considered as pharmaceutically acceptable carriers. In this regard, the thermodynamic study of the physicochemical characteristics of acid–base interaction in aqueous solutions of these biologically active substances is an extremely important problem [1–5]. As an object of study, we chose glycyl-D-phenylalanine, a dipeptide that is part of the Dalargin drug preparation (tyrosyl-D-alanyl-glycyl-phenylalanyl-leucyl-arginine diacetate). It has an antisecretory activity, promotes the activation of reparative processes, and accelerates the healing of defects in the mucous membrane of the gastrointestinal tract. This work is a continuation of our studies of acid–base interaction in aqueous solutions of various bioligands [6–8].
The acid–base properties of glycyl-D-phenylalanine (HL±) depend strongly on the pH of the medium due to its dipolarity. In an aqueous solution, glycyl-D-phenylalanine dissociates according to the following scheme:

Protolytic equilibria in an aqueous solution of glycyl-D-phenylalanine can be described by the equations
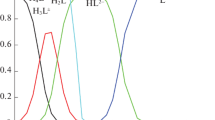
In a neutral environment, the dipeptides are in the zwitterionic form НL±. The fractional distribution of H2L+, HL±, and L– species at different pH values (Fig. 1) indicates the possibility of independent determination of the thermal effects of the dissociation of the cationic acid and zwitterion of the dipeptide (processes (1) and (2)). It follows from the diagram that only HL± and H2L+ are present in solution at pH of 1–4; only L– and HL± are present at pH of 7–11; and all the three species, at pH of 4–7. Based on the equilibrium diagram in an aqueous solution of the peptide, the concentration conditions for the calorimetric experiment were chosen.
The equilibrium constants of these processes K1 and K2, respectively, were found quite reliably [9–14]; the literature data are presented in Table 1. These studies were performed at various ionic strengths against the background of different supporting electrolytes. Therefore, in order to compare and analyze the literature data, it is necessary to find the most probable values of dissociation constants for each value of ionic strength. The constants thus obtained were extrapolated to zero ionic strength by the least squares method using an equation with one individual parameter [15].
The aim of this work was direct calorimetric determination of the thermal effects of dissociation and neutralization of glycyl-D-phenylalanine in the presence of various electrolytes at several values of ionic strength and the calculation of the corresponding standard values.
EXPERIMENTAL
Glycyl-D-phenylalanine (ReaKhim, 98.8% main component) was used without additional purification. The calorimetric measurements were performed in the ampule of a calorimeter with an isothermal shell and a KMT-14 thermistor temperature sensor, while automatically recording the temperature changes over time [16, 17]. The operation of the calorimeter was checked according to the thermal effect of the potassium chloride dissolution in water. The agreement of the experimentally measured values with the most reliable literature data [18] indicated the absence of noticeable systematic errors in the operation of the calorimeter. To maintain the given value of the ionic strength of the solution, potassium and sodium nitrates and sodium chloride were used, which were prepared from recrystallized reagents of reagent grade. The samples of solutions were weighed on a VLR-200 balance with an accuracy of 2 × 10–4 g.
When determining the thermal effect of dissociation of H2L+, it was taken into account that the following processes occur in the system:
The following procedure was used to determine the thermal effect of proton addition to the carboxyl group of glycyl-D-phenylalanine. A 0.01 M solution of the peptide (pHinit 3.6) served as a calorimetric liquid. The pH was created by introducing an exact amount of the supporting electrolyte. The ampule contained accurate samples of solutions of HNO3 (with a concentration of 0.5903 mol/kg, 0.6131 M) or HCl (with a concentration of 0.9902 mol/kg, 1.0355 M). After mixing, the pH of the solutions was close to 2.0. In this way the enthalpies of interaction (ΔmixH1) of a mineral acid solution with a peptide solution were determined (Table 2). In addition, the enthalpies of dilution (ΔdilH1) were determined in a separate series of experiments. The ampule contained solutions of mineral acids (of the same concentration as in the first series of the experiment when determining (ΔmixH1), and supporting electrolyte solutions served as a calorimetric liquid. The measurements were performed at ionic strengths of solution of 0.5, 0.75, and 1.0 M. The experimental data are given in Table 2.
When determining the thermal effects of peptide neutralization in the system, the following processes were considered:
The thermal effect of glycyl-D-phenylalanine dissociation at the second step was studied at pH in the range from 10.2 to 8.1. For this, we measured the thermal effects (ΔmixH2) of the interaction of a 0.02 M glycyl-D-phenylalanine solution with solutions of carbonate-free hydroxides NaOH, LiOH, and KOH, prepared by the standard procedure from reagents of reagent grade. When determining the enthalpies of dilution (ΔdilH2), pH of the supporting electrolyte solution was set equal to the initial pH of the peptide solution; thus, dilution occurred without changing the equilibrium composition of the system. To maintain the given ionic strength, exact amounts of NaCl, NaClO4, NaNO3, KNO3, and LiNO3 were introduced in the solution. In the initial solution, pH 8.1 was created by adding the required amount of the corresponding alkali. The experimental data in the form of arithmetic means over three or four experiments are given in Table 3.
RESULTS AND DISCUSSION
The change in enthalpy during the acid dissociation of the carboxyl group of glycyl-D-phenylalanine ΔdisН(H2L+) was found by the equation
where ΔmixН1 is the thermal effect of the interaction of a mineral acid solution with a peptide solution; ΔdilH1 is the change in the enthalpy of dilution of a mineral acid solution in a supporting electrolyte solution; and α1 is the completeness of protonation of HL±.
The equilibrium composition of solutions before and after the calorimetric experiment was calculated using the universal KEV program [19]. The calculation showed that nitric and hydrochloric acids react with the zwitterion according to a scheme that is the reverse of process (1) by 70–80%. The thermal effects of dissociation of glycyl-D-phenylalanine at the first step at different values of ionic strength are given in Table 2.
The thermal effect of dissociation of the betaine proton of the peptide (ΔdisH(HL±)) was calculated by the equation
where Δ[L–], Δ[H2L+], and Δ[H+] is the difference between the final and initial equilibrium concentrations of the corresponding species; \({{m}_{{{\text{H}}{{{\text{L}}}^{{{ \pm }}}}}}}\) is the mass of the peptide; and \({{C}_{{{\text{H}}{{{\text{L}}}^{{{ \pm }}}}}}}\) is the peptide concentration (M).
Since the completeness of the peptide neutralization process was 99.9%, and, consequently, the contributions of other terms can be neglected, Eq. (10) can be written in a simplified form:
where ΔmixH2 is the thermal effect of the interaction of the peptide solution with the alkali solution; ΔdilH2 is the thermal effect of dilution of the peptide solution in the supporting electrolyte solution; α2 is the completeness of peptide neutralization; and ΔНw is the thermal effect of water dissociation in the supporting electrolyte solution.
The value of ΔНw was taken from [20] for the supporting electrolytes used in our work. The error was determined as the standard deviation of the average value from three or four parallel experiments.
The enthalpies of stepwise dissociation of glycyl-D-phenylalanine at zero ionic strength were found using the equation with one individual parameter [15]:
where ΔН and ΔН° is the change in enthalpy at the final value of the ionic strength and at I = 0, respectively; Ψ(I) is the theoretically calculated function of the ionic strength; ΔZ 2 is the difference in the squares of the charges of the reaction products and the initial components; and b is the empirical coefficient.
For processes with ΔZ 2 = 0, Eq. (12) takes the form
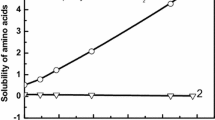
The results of graphical processing of the obtained experimental data on the thermal effects of dissociation and neutralization of glycyl-D-phenylalanine are presented in Figs. 2 and 3. It can be seen that the points quite satisfactorily fit the straight lines for each of the supporting electrolytes NaCl, NaClO4, NaNO3, KNO3, and LiNO3, and that the segments cut off by these straight lines on the y axis give the values of the standard thermal effect. The most probable values of standard thermal effects are found as the arithmetic mean of the ΔН° values obtained for each of the supporting electrolytes.
According to Fig. 2, an increase in the concentration of NaCl, NaNO3, and KNO3 causes some increase in the endothermicity of acid dissociation of the dipeptide. However, even at I = 1.0 mol/L, the difference in the enthalpies of dissociation of glycyl-D-phenylalanine at the first step, found against the “background” of NaCl and KNO3, is only 300 J/mol. Thus, it can be noted that for both aliphatic amino acids and dipeptides, there is no significantly different effect of the nature of supporting electrolytes on the thermal effect of acid dissociation.
The standard thermodynamic characteristics of the stepwise dissociation of glycyl-D-phenylalanine are presented in Table 4. It was noted [21] that the equilibrium constants related to the final value of ionic strength are currently often used to calculate the change in the standard Gibbs energy and, further, to calculate the change in entropy. Numerous literature data also show that the standard thermodynamic functions are often calculated from the concentration constants found at ionic strengths of 0.1, 0.5, 3.0, etc. created by different supporting electrolytes.
We recalculated the constants for zero ionic strength of solution using the Davies equation for 298.15 K. These are exactly the parameters (I = 0.0 M and T = 298.15 K) that characterize the standard state, to which the entropies calculated by us refer.
The stepwise dissociation of peptides, as well as aliphatic amino acids, is accompanied by a negative and small change in entropy [22–24]. This significantly distinguishes the dissociation of amino acids in an aqueous solution from the ionization of weak organic acids [25, 26].
REFERENCES
M. S. Sukhareva, P. M. Kopeykin, M. S. Zharkova, and O. V. Shamova, Med. Acad. J. 19, 180 (2019). https://doi.org/10.17816/MAJ191S1180-181
A. D. Neklyudov and E. K. Denyakina, Appl. Biochem. Microbiol. 40, 370 (2004). https://doi.org/10.1023/B:ABIM.0000033913.63770
A. N. Inozemtsev, D. S. Berezhnoy, T. N. Fedorova, and S. L. Stvolinsky, Dokl. Biol. Sci. 454, 16 (2014). https://doi.org/10.1134/S0012496614010177
A. K. Brel, S. V. Lisina, and Y. N. Budaeva, Russ. J. Org. Chem. 57, 540 (2021). https://doi.org/10.1134/S1070428021040060
E. Yu. Tyunina, V. P. Barannikov, V. V. Dunaeva, and A. V. Krasnov, Russ. J. Phys. Chem. A 96, 696 (2022). https://doi.org/10.1134/S003602442204032X
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and I. A. Skvortsov, J. Therm. Anal. Calorim. 130, 457 (2017). https://doi.org/10.1007/s10973017-6134-6
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, P. D. Krutov, and R. A. Romanov, Russ. J. Phys. Chem. A 96, 1698 (2022). https://doi.org/10.1134/S0036024422080131
A. I. Lytkin, O. N. Krutova, E. Yu. Tyunina, V. V. Chernikov, Yu. V. Mokhova, and E. D. Krutova, Russ. J. Phys. Chem. A 95, 2051 (2021). https://doi.org/10.1134/S0036024421100162
M. Shoukry, E. Khairy, and A. El-Sherif, Trans. Met. Chem. 27, 656 (2002). https://doi.org/10.1023/A:1019831618658
M. Nair and G. Subbalakshmi, Indian J. Chem. 39A, 468 (2000).
C. Agoston, T. Jankowska, and I. Sovago, J. Chem. Soc. Dalton Trans., 3295 (1999). https://doi.org/10.1039/a904000e
A. Kufelnicki, Pol. J. Chem. 66, 1077 (1992).
M. Jezowska-Bojczuk, H. Kozlowski, I. Sovago, et al., Polyhedron 10, 2331 (1991). https://doi.org/10.1016/S0277-5387(00)86157-4
G. Brookes and L. Pettit, J. Chem. Soc. Dalton Trans., 2106 (1975). https://doi.org/10.1039/dt9750002106
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Nauka, Moscow, 1982) [in Russian].
O. N. Krutova, A. I. Lytkin, V. V. Chernikov, et al., J. Mol. Liq., 116773 (2021). https://doi.org/10.1016/j.molliq.2021.116773
E. Tyunina, O. Krutova, and A. I. Lytkin, Thermochim. Acta 690, 178704 (2020). https://doi.org/10.1016/j.tca.2020.178704
W. B. Parcker, Thermal Properties of Aqueous Uni-univalent Electrolytes (NSRDS-NBS, Washington, 1965), Vol. 2, p. 342.
A. N. Meshkov and G. A. Gamov, Talanta 198, 200 (2019). https://doi.org/10.1016/j.talanta.2019.01.107
V. P. Vasil’ev and L. D. Shekhanova, Zh. Neorg. Khim. 19, 2969 (1974).
V. P. Vasil’ev, Russ. J. Coord. Chem. 30, 68 (2004).
S. N. Gridchin, Russ. J. Gen. Chem. 85, 810 (2015).
L. A. Kochergina, V. P. Vasil’ev, and O. N. Krutova, Russ. J. Phys. Chem. A 82, 348 (2008).
A. I. Lytkin, V. V. Chernikov, and O. N. Krutova, Russ. J. Phys. Chem. A 90, 1530 (2016). https://doi.org/10.7868/S0044453716080173
V. P. Vasil’ev and L. A. Kochergina, Zh. Fiz. Khim. 41, 1287 (1967).
L. A. Kochergina, V. P. Vasil’ev, D. V. Krutov, and O. N. Krutova, Russ. J. Phys. Chem. A 82, 565 (2008).
Funding
This study was performed at the Research Institute of Thermodynamics and Kinetics of Chemical Processes, Ivanovo State University of Chemistry and Technology, under the government contract (project no. FZZW-2023-0008) using the resources of the Multiaccess Center of the Ivanovo State University for Chemical Technology. The study was supported by the Ministry of Science and Higher Education of Russia, project no. 075-15-2021-671.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Kudrinskaya
Rights and permissions
About this article
Cite this article
Krutova, O.N., Bazanov, M.I., Chernikov, V.V. et al. Effect of the Nature of Supporting Electrolyte on the Thermodynamic Parameters of the Stepwise Dissociation of Glycyl-D-Phenylalanine in Aqueous Solution. Russ. J. Phys. Chem. 97, 1901–1906 (2023). https://doi.org/10.1134/S003602442309011X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442309011X