Abstract
Expressions are obtained for the Gibbs energy of solid uranium diboride UB2 at T = 0–2300 K in the standard element reference system. They are established to describe the experimental data on heat capacity and heat content throughout the range of temperatures with a single dependence. The weighted sum of the Einstein functions with no polynomial contribution is shown to approximate the limit behavior of the heat capacity at T = 1–5 K. A simplified dependence with six parameters is proposed for the Gibbs energy at T = 200–2000 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Uranium diboride UB2 is of interest as a component of promising nuclear fuels resistant to accidents (accident tolerant fuels) [1]. Studies of its thermodynamic properties are important both for optimizing the conditions for fuel production and for predicting phase and chemical equilibria with its participation. The thermodynamic model of a uranium–boron binary system in [2] allows us to obtain the phase diagram and the properties of all binary compounds in it: UB2, UB4, and UB12. It belongs to the second-generation CALPHAD models; i.e., it is based on polynomial functions and can be used only when T ≥ 298.15 K. A possible alternative is the third-generation CALPHAD models first proposed in 1995 [3]. The Einstein or Debye functions are used in these models to approximate isobaric heat capacity Cp, ensuring their applicability down to 0 K and the possibility of extrapolating them to both low and high temperatures. Voronin and Kutsenok [4] and Jacobs et al. [5] independently proposed using the weighted sum of several Einstein functions to achieve high accuracy in approximating the heat capacity of complex substances in a wide range of temperatures. This model was supplemented with a polynomial in [6] and used to approximate the heat capacities of graphite and diamond.
There are experimental data on isobaric heat capacity Cp [7] and heat content HT − H298.15 [8, 9] for solid uranium diboride UB2 that cover the 1.1–2300 K range of temperatures. A brief list of these is presented in Table 1. All were obtained for samples of composition UB1.979. To move to thermodynamic functions of stoichiometric UB2, we used factor 3/2.979 ≈ 1.007 proposed in [7]. Values of the thermodynamic functions of UB2 at T = 298.15 K, obtained from experimental data on heat capacity, were recommended in that work: \(S_{{298.15}}^{ \circ } = 55.51 \pm 0.11\) J/(mol K), \(H_{{298.15}}^{ \circ } - H_{0}^{ \circ } = 8880 \pm 17\) J/mol, and \(C_{{p,298.15}}^{ \circ }\) = \(55.76 \pm 0.11\) J/(mol K).
The enthalpy of formation of uranium diboride \({{{{\Delta }}}_{{\text{f}}}}H_{{298.15}}^{ \circ }({\text{U}}{{{\text{B}}}_{2}}) = - 164.43 \pm 17\) kJ/mol (‒39.3 \( \pm \) 4.0 kcal/mol) [7] was obtained by burning a UB2 sample in a fluorine atmosphere inside a calorimetric bomb, and using literature data on the enthalpies of formation of UF6 and BF3.
The temperature dependence of the Gibbs energy for UB2 at T = 298.15–2300 K [2] in the standard element reference system is presented along with experimental data. The formulas are given in terms of U1/3B2/3:
where HSER is the reference level, i.e., the enthalpies of formation of simple substances (stable allotropic modifications) at T = 298.15 K and p = 1 bar. The generalized GT – HSER expression for an individual compound is
where \({{{{\Delta }}}_{{\text{f}}}}H_{{298.15}}^{ \circ }\) is the standard enthalpy of formation. For UB2, we used the value obtained for it in [7] (see above). Disadvantages of this polynomial model are the use of two different dependences for T = 298.15–1600 and >1600 K, along with its unsuitability for calculating Cp at T < 298.15 K. Entropy \(S_{{298.15}}^{ \circ }~\) = 54.525 J/(mol K) was used as is (i.e., it cannot be obtained from the Cp(T) polynomial dependence).
No thermodynamic models that allow us to calculate isobaric heat capacity of UB2 at T = 0–298.15 K are described in the literature. The following approximation of low-temperature Cp for T < 4.2 K was proposed in [7]:
An approximation of the heat capacity anomaly of solid UB2 at T = 40–100 K, made using a two-level Schottky model, was proposed in the same work. Unfortunately, these two models do not cover the entire 0–298.15 K range of temperatures and cannot be used to calculate \(S_{{298.15}}^{ \circ }\). While the experimental heat capacities were approximated in [7] by polynomial dependences, the dependences themselves are not given.
The aim of this work was to obtain the temperature dependences of the Gibbs energy of solid uranium diboride UB2, based on the third-generation CALPHAD models and the available experimental data on isobaric heat capacity, heat content, and enthalpy of formation.
CALCULATIONS
Experimental data on heat capacity and heat content were approximated using a model that included a weighted sum of Einstein functions and a polynomial contribution:
where T0 = 298.15 K; R is the universal gas constant; m is the number of Einstein functions; and \({{\alpha }_{i}}\), \({{\theta }_{i}}\), a1, and a2 are model parameters. This model was proposed in [6] and obtained by adding a polynomial to the model of isobaric heat capacity proposed by Voronin and Kutsenok in [4] and based on the weighted sum of Einstein functions. The polynomial of Eq. (5) differs from the AT + BT4 expression used in [6] by dimensionless coefficients a1 and a2.
The expressions for entropy and heat content can be obtained by integrating Eq. (6):
The expression for the Gibbs energy in the standard element reference system can be obtained by substituting Eqs. (7)–(10) into Eq. (3):
Four versions of the above model were used to approximate experimental data.
The first was model Ein with no polynomial (i.e., \({{a}_{1}} = {{a}_{2}} = 0\) in Eqs. (5), (7), (9), and (11)), the parameters of which were obtained using all of the experimental data in Table 1.
The second was model EinLT with no polynomial. Only data on interval T = 0–1486 K were used in optimizing its parameters.
The third was model EinPoly with a polynomial (i.e., \({{a}_{1}} \ne 0\) and \({{a}_{2}} \ne 0\)) based on the same data as model Ein.
The fourth was simplified model Ein2 with two Einstein functions and a polynomial based on the data for T = 200–2300 K, along with values \(S_{{298.15}}^{ \circ }\) and \(H_{{298.15}}^{ \circ } - H_{0}^{ \circ }\) obtained using model Ein.
The model parameters were optimized using nonlinear least squares in the CpFit program [10]. An objective function based on a weighted sum of the squares of relative deviations was used for the first three models:
where indices calc and exp denote calculated and experimental values, and ωС and ωH are the statistical weights for isobaric heat capacities and heat contents, respectively: ωС = 1 for T ≥ 5 K and ωС = 0.25 for T < 5 K. Unit statistical weights of ωH = 1 were used in all models except Ein2, where ωH = 1 and 0.1 for points from [8] (T = 479–1486 K) and [9] (T = 1303–2300 K), respectively. For simplified model Ein2 with two Einstein functions and a polynomial, experimental data were used exclusively for T > 100 K. The reproducibility of \(S_{{298.15}}^{ \circ }\) and \(H_{{298.15}}^{ \circ } - H_{0}^{ \circ }\) was ensured by introducing two additional terms into the objective function (i.e., using a new RSS2 objective function):
where RSS is the objective function described by Eq. (13), and superscripts calc and ref refer to values obtained from models Ein2 and Ein, respectively. When optimizing the parameters of model Ein2, additional condition \(\sum\nolimits_i^{} {{{\alpha }_{i}}} = {{N}_{{{\text{atoms}}}}}\) = 3 implemented in the CpFit program by a change of variables was used as well:
where \({{\xi }_{1}} \in \left[ {0;\,\,1} \right]\) is the parameter to be optimized, and \({{N}_{{{\text{atoms}}}}} = 3\).
Two values were used to estimate the accuracy of the experimental data approximation: the standard deviation and the normalized median absolute deviation. They were calculated for the absolute and relative deviations as
where \(Y\) is isobaric heat capacity Cp or heat content \({{H}_{T}} - {{H}_{{298.15}}}\), and \({{{{\Phi }}}^{{ - 1}}}(x)\) is the inverse cumulative distribution function for standard normal distribution \(1{\text{/}}{{{{\Phi }}}^{{ - 1}}}(0.75) \approx 1.483\). A more detailed description of these estimates of the accuracy of approximation, including the rationale for using normalizing factor 1.483, was given in [11] on the use of the CpFit program for compiling databases.
RESULTS AND DISCUSSION
The resulting sets of parameters for Eqs. (5)–(12) are presented in Table 2. The parameters below were obtained for simplified model Ein2:
Tables 3 and 4 show the accuracy of approximating experimental data on isobaric heat capacity [7] and heat content [8, 9] using different thermodynamic models: those obtained in this work and taken from [1]. The two temperature ranges in the data from [7] correspond to samples obtained by isoperibol and adiabatic calorimetry (see Table 1).
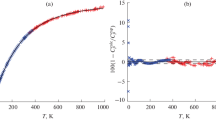
Figures 1 and 2 show results from approximating experimental data on isobaric heat capacity and heat content. The temperature dependences of Cp and HT – H298.15 are shown in Figs. 1a and 2a, and the corresponding scatter plots are shown in Figs. 1b and 2b.
Results from approximating experimental values of the isobaric heat capacity of UB2: (a) dependences of Cp on T; (b) dependences of relative deviations \(\varepsilon {{C}_{p}} = 100(C_{p}^{{{\text{expt}}}} - C_{p}^{{{\text{calc}}}}){\text{/}}C_{p}^{{{\text{expt}}}}\) on T. The solid, dashed, dashed-and-dotted, and dotted lines are models Ein, EinPoly, Ein2, and Poly, respectively. Dots are experimental values of Cp from [7]; rings and triangles are data from isoperibol and adiabatic calorimetry, respectively.
Results from approximating experimental values of the heat content of UB2: (a) the dependence of \({{\Delta }}H = ({{H}_{T}} - {{H}_{{{{T}_{0}}}}}){\text{/}}(T - {{T}_{0}})\) on T, T0 = 298.15 K; (b) the dependence of relative deviations \(\varepsilon {{\Delta }}H = 100({{\Delta }}{{H}^{{{\text{expt}}}}} - {{\Delta }}{{H}^{{{\text{calc}}}}}){\text{/}\Delta }{{H}^{{{\text{expt}}}}}\) on T. The bold, fine, dashed, dashed-and-dotted, and dotted lines show data obtained using models Ein, EinLT, EinPoly, Ein2, and Poly, respectively. Dots are experimental data obtained via drop calorimetry; diamonds and squares are values from [8] and [9], respectively.
Files of the initial data and obtained model parameters are available at https://doi.org/10.17632/3vkpz6nfff.1 as the Mendeley Data Set for the CpFit and GNU Octave programs for plotting and tables.
Model Ein with nine Einstein functions and no polynomial with 18 optimized parameters turned out to be the most accurate of those obtained in this work. In it, \(\sum\nolimits_i^{} {{{\alpha }_{i}}} \gg {{N}_{{{\text{atoms}}}}} = 3\). This because with no polynomial, difference Cp – CV, the anharmonicity of lattice vibrations, and the growth of Cp before melting are approximated by Einstein functions with values of \({{\alpha }_{i}}\) and \({{\theta }_{i}}\), which have no explicit physical meaning. This was observed in [10] for uranium dioxide UO2.
Adding a polynomial to model EinPoly allowed us to approximately meet condition \(\sum\nolimits_i^{} {{{\alpha }_{i}}} \approx {{N}_{{{\text{atoms}}}}}\) for UB2 and reduce the number of Einstein functions to five, and the number of model parameters to 12 (Table 2). It also improved the limit behavior of the model at T < 1 K (see Fig. 1a). Models Ein and EinPoly in this case have comparable accuracy throughout the T = 1–2300 K range of temperatures, and the differences when T < 1 K do not affect the values of \(S_{{298.15}}^{ \circ }\) and \({{H}_{T}} - {{H}_{{298.15}}}\) appreciably. Condition \(\sum\nolimits_i^{} {{{\alpha }_{i}}} \approx {{N}_{{{\text{atoms}}}}} = 3\) was met approximately for model EinLT by excluding data on heat content when T > 1486 K. This allowed us to reduce the number of Einstein functions to eight, and the number of parameters to 16. It also reduced the accuracy of approximating the experimental values of HT – H298.15 from [8] (T = 1303–2300 K).
Models Ein, EinLT, and EinPoly obtained in this work describe the heat capacity of UB2 more accurately than the polynomial model available in [2]. They are not inferior to it in terms of approximating heat content, but the first two require more optimized parameters. The polynomial model in [2] has only 12 parameters: six for each temperature interval (see Eqs. (1) and (2)). To test the possibility of reducing their number, we constructed simplified model Ein2 with six parameters that included two Einstein functions and a polynomial. Its accuracy was comparable to models Ein, EinLT, and EinPoly at T = 200–2000 K with fewer parameters. Its accuracy fell notably outside this range (see Figs. 1, 2), but the values it produced remained physically correct.
The tabulated values of the thermodynamic functions of solid uranium diboride UB2 when T = 1–2300 K are given in Table 5. They were calculated using model Ein (i.e., the one based on the weighted Einstein function with no polynomial), since it was more accurate than the others, including the polynomial model described in [2]. The values of \(C_{{p,298.15}}^{ \circ }\), \(S_{{298.15}}^{ \circ }\), and \({{H}_{T}} - {{H}_{{298.15}}}\) obtained from all four models constructed in this work coincided within their confidence intervals. They also agreed with the data in [7].
CONCLUSIONS
Thermodynamic models based on the weighted sum of Einstein functions allowed us to approximate experimental data on the heat capacity and heat content of solid uranium diboride throughout the 1–2300 K range of temperatures. Adding a polynomial of the AT + BT4 form to dependence Cp(T) did not improve the accuracy of approximation. However, it did reduce the number of model parameters, allowed us to meet condition \(\sum\nolimits_{i~ = ~1}^m {{{\alpha }_{i}}} = {{N}_{{{\text{atoms}}}}}\) approximately, and improved the limit behavior of function Cp(T) when T → 0 K. A simplified thermodynamic model with two Einstein functions and a polynomial can be used when T = 200–2000 K. It produces physically correct heat capacities when extrapolated to high and low temperatures, along with accurate values of \(C_{{p,298.15}}^{ \circ }\), \(S_{{298.15}}^{ \circ }\), and \({{H}_{T}} - {{H}_{{298.15}}}\). Its advantages over the polynomial model of [2] are half the number of parameters, no piecewise specified functions, and physically correct behavior when extrapolated to low temperatures.
REFERENCES
J. Turner, S. Middleburgh, and T. Abram, J. Nucl. Mater. 529, 151891 (2020). https://doi.org/10.1016/j.jnucmat.2019.151891
P. Y. Chevalier and E. Fischer, J. Nucl. Mater. 288, 100 (2001). https://doi.org/10.1016/S0022-3115(00)00713-3
M. W. Chase, I. Ansara, A. Dinsdale, et al., CALPHAD 19, 437 (1995). https://doi.org/10.1016/0364-5916(96)00002-8
G. F. Voronin and I. B. Kutsenok, J. Chem. Eng. Data 58, 2083 (2013). https://doi.org/10.1021/je400316m
M. H. G. Jacobs, R. Schmid-Fetzer, and A. P. van den Berg, Phys. Chem. Miner. 40, 207 (2013). https://doi.org/10.1007/s00269-012-0562-4
S. Bigdeli, Q. Chen, and M. Selleby, J. Phase Equilib. Diffus. 39, 832 (2018). https://doi.org/10.1007/s11669-018-0679-3
H. E. Flotow, D. W. Osborne, P. A. G. O’Hare, et al., J. Chem. Phys. 51, 583 (1969). https://doi.org/10.1063/1.1672038
D. R. Fredrickson, R. D. Barnes, M. G. Chasanov, et al., High Temp. Sci. 1, 373 (1969).
D. R. Fredrickson, R. D. Barnes, and M. G. Chasanov, High Temp. Sci. 2, 299 (1970).
A. L. Voskov, I. B. Kutsenok, and G. F. Voronin, CALPHAD 61, 50 (2018). https://doi.org/10.1016/j.calphad.2018.02.001
A. L. Voskov, G. F. Voronin, I. B. Kutsenok, and N. Yu. Kozin, CALPHAD 66, 101623 (2019). https://doi.org/10.1016/j.calphad.2019.04.008
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 20-03-00575, and under the topic “Chemical Thermodynamics,” grant no. 121031300039-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares he has no conflicts of interest.
Additional information
Translated by A. Ivanov
Rights and permissions
About this article
Cite this article
Voskov, A.L. Using Third-Generation CALPHAD Models to Approximate Thermodynamic Properties of Solid Uranium Diboride. Russ. J. Phys. Chem. 96, 2105–2112 (2022). https://doi.org/10.1134/S0036024422100326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422100326