Abstract
The bimetallic Pt–Ni nano-catalysts with different platinum to nickel mass ratios supported on the carbon nanotubes (PtNi(x : y)/FCNTs-D) were successfully synthesized by the polyol method and applied to the H2 generation from ammonia borane (AB) hydrolysis. Several state-of-the-art characterization methods were performed to explore the correlation between catalytic performance and the physicochemical characterizations of the catalysts. The results show that the optimum Pt : Ni ratio is critical to enhance the H2 generation from AB hydrolysis and in this study, it is 1 : 2. The presence of carbon nanotubes enhanced the dispersion and stability of the Pt–Ni nanoparticles. The availability of more active sites and lowered binding energy of Pt0 in PtNi(1 : 2)/FCNTs-D catalyst, lead to the increased catalytic activity of H2 generation from AB hydrolysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Hydrogen, one of the cleanest fuels, has received a lot of attention [1]. However, the controlled storage and release of hydrogen is still a challenging issue [2]. In recent years, different materials have been developed for the storage of hydrogen such as metal organic framework materials (MOF-5) [3], metal hydrides (LaNi5H6, NaAlH4, LiAlH4, AlH3) [4], borohydrides [5], organic liquid storage hydrogen materials [6] and ammonia borane (AB) [7]. Among them, solid ammonia borane, is regarded as potential hydrogen storage material [8] because of high-quality hydrogen storage density (19.6%) and high stability. In addition, the hydrogen stored in AB can be released through its hydrolysis at room temperature in the presence of a suitable catalyst.
Noble metal catalysts with high catalytic activity are commonly used catalysts for the hydrolysis of AB, among them Pt is the most active metals for AB dehydrogenation [9, 10]. Chen et al. reported Pt/CNT-O-HT catalyst for catalyzing the hydrolytic dehydrogenation of AB with TOF up to 567 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\). Because of depleting Pt sources and high price, the achieved excellent results are yet not conducive to practical applications. Therefore, an effective strategy is the combination of Pt with earth abundant transition metals [11]. The synergistic effect between different metal atoms can significantly improve the activity and selectivity of catalytic reactions [12, 13]. The Pt–Ni bimetallic nano-catalyst is considered to be one of the most potential catalysts in hydrogen generation AB hydrolysis. Thence, scientific design and controllable preparation of Pt–Ni bimetallic nano-catalyst are of great significance to the practical application of hydrogen production reaction system from AB. Moreover, the metal nanoparticles are often supported on the various carriers to avoid agglomeration and deactivation. In recent years, carbon materials with unique physical and chemical properties such as porous carbon, carbon nanotubes and graphene have been widely used as excellent carriers [14, 15]. In addition to the carbon materials, the metal nanoparticles were also supported on other metal oxides such as CeO2, ZrO2, HfO2, and TiO2, has also shown a good catalytic activity in AB hydrolysis.
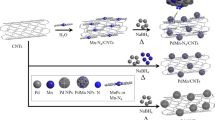
Inspired by the above-described studies, we herein report a simple and convenient method for the design and synthesis of a bimetallic Pt–Ni nano-catalysts with different platinum to nickel mass ratios supported on the carbon nanotubes. The proposed preparation protocol of the PtNi(x : y)/FCNTs-D catalysts is outlined in Fig. 1. All the freshly prepared catalysts were tested based on their catalytic activity for AB hydrolysis. In addition, the effect of temperature to the catalytic activity of selected PtNi(1 : 2)/FCNTs-D catalyst for AB hydrolysis of AB is also studied. Based on initial assessments, the bimetallic Pt–Ni nano-catalysts supported on the carbon nanotubes can significantly improve the catalytic activity and the stability of the active center of the catalyst.
EXPERIMENTAL
Commercially available materials were used without further purification. Ethanol absolute (AR), acetone (AR), nickel chloride hexahydrate (AR), ethylene glycol (AR), and NaOH (AR) were purchased from Keshi. Ammonia borane (AB, 97%) and chloroplatinic acid hexahydrate (AR) were purchased from Aladdin. Carboxylated carbon nanotubes (L = 0.5–2 μm, D = 8–15 nm) was purchased from Timesnano. Deionized water was used for the preparation of metal precursor salt solution.
The PtNi(x : y)/FCNTs-D catalysts were prepared by the polyol method (see Fig. 1). For the catalyst synthesis, carboxylated carbon nanotubes (100 mg) was added to ethylene glycol (30 mL) solution. H2P-tCl6⋅6H2O (8 mg) and NiCl2⋅6H2O (12 mg) were added to the solution of ethylene glycol (20 mL). Later, the salt solution was added dropwise to the ethylene glycol dispersion containing carboxylated carbon nanotubes under continuous stirring conditions. After it, the mixture was sonicated for 30 min and kept under overnight stirring. The resultant reaction mixture’s pH was adjusted to 11 after transferring it to 100 mL flask and kept reacting for 4 h at 160°C. Acetone was added to get precipitates and solid sample was obtained after centrifugal separation. The solid sample was multiply rinsed (3 times) by using a solvent co-mposed of acetone and ethanol, then dried overnight under vacuum at 65°C to obtain the PtNi(1 : 1)/FCNTs-D catalyst. Similarly, the catalysts with different platinum-nickel ratios were prepared by changing the amount of nickel precursor salt and abbreviated as PtNi(x : y)/FCNTs-D.
The morphology structures of samples were characterized by transmission electron microscope (Hitachi-7700 TEM, Hitachi, accelerating voltage 100 kV). The XRD patterns of various catalysts were collected from a PANalytical X-ray powder diffractometer (XRD, X’Pert PRO MPD) using CuKα radiation (λ = 1.5406 Å) over a scan range of 10°–80°. X-ray photoelectron spectroscopy (XPS) was acquired by Thermo VG ESCALAB 250 (Thermo Fisher Scientific, US) and the charge correction was performed at 284.5 eV of carbon. Fourier transform infrared spectroscopy (F-T‑IR) were collected on a WQF 520 (Beijing Ruili Analytical Instrument Co., Ltd.).
The catalytic activity tests for AB hydrolysis to hydrogen generation were carried in a device as shown in Fig. 2. 5 mg of the freshly prepared catalyst and 5 mL of deionized water was added into a 10 mL three-necked flask and was ultrasonically mixed to get uniform dispersion. Next, the three-necked flask containing the uniformly mixed dispersion was placed in a constant temperature water bath and was kept stirring. Both the left and right openings of the three-necked flask were sealed and the gas measuring device was connected through the middle opening. After the addition of AB solution, composed of 30 mg of AB and 0.2 mL of deionized water, the left opening of the three-necked flask was sealed again. The hydrogen generation rate from AB hydrolysis of AB was calculated by following equation:
where TOF is rate of hydrogen generation from AB hydrolysis (min–1), \({{n}_{{\text{H}}}}_{{_{2}}}\) is molar mass of the volume of hydrogen generation (mol), \({{n}_{{{\text{catalyst}}}}}\) is molar mass of the catalyst (mol), t is reaction time (min). The term TOF was used to evaluate and compare the catalytic activity of PtNi(x : y)/FCNTs-D catalyst. It refers to the molar amount of hydrogen generation per unit time per unit of the catalyst.
The activation energy test of hydrogen generation from AB hydrolysis was used to measure the difficulty of the reaction in the presence of the catalyst. The hydrogen generation rate constant k was measured from the data obtained from the reaction under a series of water bath reaction temperature. The activation energy (Ea) was measured by using following equation:
where k is hydrogen generation rate constant, A is pre-reference factor, Ea is activation energy (kJ mol–1), R is ideal gas constant (8.314 J mol–1 K–1), T is reaction temperature (K).
The repeatability and durability tests for hydrogen generation were conducted by using five repeated cycle of catalytic activity. After each catalyst activity test cycle for hydrogen generation, the catalyst was washed and used again to study the hydrogen generation at 298 K.
RESULTS AND DISCUSSION
The morphology, the average Pt particle size and its distribution in all the PtNi(x : y)/FCNTs-D catalyst were studied by using transmission electron microscopy (TEM) (see Figs. 3a–3d and 3e–3h). It was found that highly dispersed nanoparticles were clearly loaded on the carboxylated carbon nanotubes. The average Pt particle size distribution in the PtNi(1 : 0)/FCNTs-D, PtNi(1 : 1)/FCNTs-D, PtNi(1 : 2)/FCNTs-D, and PtNi(1 : 4)/FCNTs-D catalysts was 1.35 nm (see Fig. 3e), 1.85 nm (see Fig. 3f), 1.95 nm (see Fig. 3g), and 1.95 nm (see Fig. 3h), respectively. The overall range of the average Pt particle size was in the range from 1.35 to 1.95 nm. The TEM analysis showed that the average Pt particle size was increased with the increase in Ni amount up to Pt : Ni ratio of 1 : 2. Upon further increase in Ni amount, for instance in case of PtNi(1 : 4)/FCNTs-D catalyst, the average Pt particle size was the same as to PtNi(1 : 2)/FCNTs-D catalyst. It showed that after a certain Pt to Ni ratio, there was no impact of Ni amount to the Pt particle size.
The X-ray diffraction (XRD) studies (see Fig. 4) showed two peaks at 25.8° and 42.8°, correspond to the (002) and (101) graphitic carbon reflections. Except afore mentioned two peaks, none of the analyzed catalytic sample (PtNi(x : y)/FCNTs-D at x : y = 1 : 1, 1 : 2, and 1 : 4) had shown any pure platinum, pure nickel and/or platinum-nickel alloy phase peaks.
To investigate the possible oxidation states and their association to the catalytic activity, all the studied catalysts were analyzed by using X-ray photoelectron spectroscopy (XPS). The results were shown in Fig. 5 and Table 1. Figure 5a was an overall comparison of the identified peaks of the all the four catalysts (PtNi(x : y)/FCNTs-D at x : y = 1 : 0, 1 : 1, 1 : 2, and 1 : 4). The peaks at 72.0, 284.5, 532.16, and 856.54 eV correspond to Pt 4f, C 1s, O 1s, and Ni 2p [16], respectively. To understand the different Pt and Ni oxidation states, each Pt and Ni corresponding peak in PtNi(x : y)/FCNTs-D catalyst was deconvoluted and results were shown in Figs. 5b and 5c. The peaks at 71.80 eV (4f7/2) and 75.15 eV (4f5/2) corresponded to Pt0, peaks at 72.70 eV (4f7/2) and 76.30 eV (4f5/2) corresponded to Pt2+, peaks at 74.90 eV (4f7/2) and 78.10 eV (4f5/2) corresponded to Pt4+. The peaks at 69 eV corresponded to Ni 3p (assigned to Ni(OH)2 [17]), peaks at 852.35 eV (2p3/2) and 869.75 eV (2p1/2) corresponded to Ni0 and peaks at 856.65 eV (2p3/2) and 874.85 eV (2p1/2) corresponded to Ni2+. The overall comparison of present oxidation states of Pt and Ni were summarized in Table 1. Interestingly, the Ni2+ peaks were weighted 96% of the total other Ni peaks. In addition, with the increase in Ni amount in catalyst recipe, the peak position of Pt0 began to shift to lower binding energy (for instance, see the Ni peaks in PtNi(1 : 2)/FCNTs-D catalyst (Fig. 5c). In all studied catalytic samples, the Pt0 peaks was accounted ~50% of the total peak—a clear indication of Pt metal presence in its simplest form. In summary, the Pt0, Pt2+, Pt4+, and Ni2+ species were found in PtNi(x : y)/FCNTs-D catalysts.
Figure 6a was the comparison of catalytic activity and the amount of hydrogen generation (in terms of TOF) from AB hydrolysis of all the studied PtNi(x : y)/FCNTs-D catalysts at room temperature (298 K). The catalytic activity of PtNi(1 : 0)/FCNTs-D was 165 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\) (see Fig. 6b), which was similar to the catalytic activity of the pure platinum catalyst reported previously [18]. Whereas, the catalytic activity of the PtNi(1 : 1)/FCNTs-D catalyst was 476 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\), which was far higher as to that of PtNi(1 : 0)/FCNTs-D catalyst. The catalytic activity of the PtNi(1 : 2)/FCNTs-D and the PtNi(1 : 4)/FCNTs-D catalysts was 597 and 377 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\), respectively. In comparison, the catalytic activity of the PtNi(1 : 2)/FCNTs-D was the highest as to the other studied PtNi(x : y)/FCNTs-D catalysts. It clearly showed that the presence of Ni in the catalyst composition favorably impacted to enhance the catalytic activity of PtNi(x : y)/FCNTs-D catalyst. However, an optimum Pt : Ni was key aspect and in this study it was 1 : 2. Beyond this ratio, the catalytic activity was lowered.
To study the effect of reaction temperature to the catalytic activity and amount of hydrogen generation, the best PtNi(1 : 2)/FCNTs-D catalyst and was tested further at different reaction temperatures of 298, 303, 308, and 313 K (see Fig. 7a). For each reaction temperature, the value of hydrogen generation rate constant k was calculated. The value of activation energy Ea was calculated using Arrhenius equation (see Fig. 7b). The obtained results were summarized in Fig. 7b.
The catalytic activity performance comparison of best PtNi(1 : 2)/FCNTs-D catalyst to the reported available literature was summarized in Table 2. Based on it, the PtNi(1 : 2)/FCNTs-D catalyst need lowest activation energy (32 kJ/mol) in comparison to the Pt/CNTs-O-HT, PEI–GO/Pt0.17Co0.83, Ni0.33@Pt0.67/C, Co0.32@Pt0.68/C, and STA-Pt/CNT catalysts. The lowered Ea in AB hydrolysis for the PtNi(1 : 2)/FCNTs-D catalyst was an indication Pt : Ni ratio in the catalyst composition assisted to shift the thermodynamic equilibrium to lower side, which favorably promoted the catalytic reaction [19]. In general, the PtNi(1 : 2)/FCNTs-D catalyst had shown highest amount hydrogen generation from AB hydrolysis at 298 K.
To investigate the repeatability, the best PtNi(1 : 2)/FCNTs-D catalyst was tested repeatedly for five repeats(R1, R2, R3, R4, and R5). The amount of H2 generation in R1, R2, R3, R4, and R5 was 597, 372, 354, 290, and 287 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\), respectively (see Fig. 8). After the first repeat (R2), the amount of H2 generation from AB hydrolysis decreased to 62%. After the fifth repeat (R5), the amount of H2 generation from AB hydrolysis reduced to 48% to the initial catalytic activity. However, even after fifth repeat cycle (R5), the catalytic activity of the best PtNi(1 : 2)/FCNTs-D catalyst was higher as to the catalytic activity of the pure platinum catalyst (PtNi(1 : 0)/FCNTs-D). In general, the catalytic gradually decreased with the increase in the number of repeat test. The decrease catalytic activity for H2 generation form AB hydrolysis was due to change in morphology intertwined of carboxylated carbon nanotubes and the agglomeration of the nanoparticles due to their shedding during cleaning process (see Fig. 9). To investigate it further, an infrared analysis of PtNi(1 : 2)/FCNTs-D was performed before and after the AB hydrolysis (see Fig. 10). It was found that the used catalyst had many new characteristic peaks. Among new peaks, the peaks at 3200–3400 cm–1 corresponded to O–H stretching peak, the peak at 1450 cm–1 corresponded to B–O stretching peak, the peak at 780 cm–1 corresponded to B–O bending peak [25]. The peaks were attributed to the \({\text{BO}}_{2}^{ - }\) and/or other by-products during AB hydrolysis.
In addition to the repeatability, the PtNi(1 : 2)/FCNTs-D catalyst was tested for its durability for H2 generation from AB hydrolysis. The durability of the best catalyst was tested 5 times (D1, D2, D3, D4, and D5). The results were shown in Fig. 11. After the second reaction (D2), the catalytic activity remained almost the same as to the first reaction (D1). However, the catalytic activity was decreased slightly in the third reaction (D3; TOF = 457 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\)), which was about 77% of the initial activity. After the fifth cycle (D5), the amount of H2 produced was 304 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{2}}}}}\) min–1 \({\text{mol}}_{{{\text{pt}}}}^{{ - 1}}\)– approximately to 51% of the initial catalytic activity, which was still higher than the activity of the pure platinum catalyst PtNi(1 : 0)/FCNTs-D.
Based on the combined with TEM and XRD analysis, it was clear that bimetallic Pt–Ni nano-catalysts were successfully supported on the carbon nanotubes. None of the prepared PtNi(x : y)/FCNTs-D catalysts showed pure platinum phase or pure nickel phase or platinum-nickel alloy. This attributed to the small sized nanoparticles loaded on the carboxylated carbon nanotubes and/or amorphous shape particles. The catalyst PtNi(1 : 2)/FCNTs-D showed the highest catalytic activity in comparison to the PtNi(1 : 0)/FCNTs-D, PtNi(1 : 1)/FCNTs-D, and PtNi(1 : 4)/FCNTs-D catalyst. According to the available literature and experimental results, this phenomenon could be governed by the Sabatier principle. Due to the availability of the best free adsorption energy on the catalytic surface either to reactants and/or reaction intermediates, thus it exhibited the best catalytic activity [26]. In addition, if the binding energy of the intermediate was too weak, it would be difficult to activate the substrate on the catalyst surface and vice-versa. According to the XPS characterization, there an electron transfer took placed from Ni to Pt atoms, which caused the chemical shift of Pt element to move negatively. When the amount of nickel precursor was increased to Pt : Ni = 1 : 2, the binding energy of Pt0 decreased, and the catalytic activity got increased. Whereas, after increasing Pt : Ni ration to 1 : 4, the binding energy of Pt0 began to increase, and due to which the catalytic activity was decreased. It indicated that the lower the binding energy of Pt0, the higher the catalytic activity, and the catalyst had the best free energy of adsorption. Based on TEM and IR analysis, the decrease in catalytic activity of PtNi(1 : 2)/FCNTs-D after its multiple use could be due to the agglomeration of the nanoparticles, which lead to the lesser availability of the active sites.
CONCLUSIONS
In summary, a facile method for the preparation of bimetallic Pt–Ni nano-catalysts supported on the carbon nanotubes is beneficial to improve the catalytic activity for hydrogen generation from AB hydrolysis. In addition, the presence of carbon nanotubes in the catalyst composition improves the dispersion and stability of the nanoparticles. The availability of more active sites and lowered binding energy of Pt0 in PtNi(1 : 2)/FCNTs-D catalyst, lead to the increased catalytic activity of H2 generation from AB hydrolysis at room temperature as to the other studied PtNi(1 : 0)/FCNTs-D, PtNi(1 : 1)/FCNTs-D, and PtNi(1 : 4)/FCNTs-D catalysts. In brief, a new synthesis strategy to design bimetallic catalysts for highly efficient hydrogen generation is the key aspect of this study.
REFERENCES
Y. Du, Y. Shen, Y. Zhan, et al., Chin. Chem. Lett. 28, 1746 (2017).
L. M. Kustov, A. N. Kalenchuk, and V. I. Bogdan, Russ. Chem. Rev. 89, 897 (2020).
S. Yu, G. Jing, S. Li, et al., Int. J. Hydrogen Energy 45, 6757 (2020).
G. Sandrock, J. Reilly, J. Graetz, et al., J. Alloys Compd. 421, 185 (2006).
A. Züttel, P. Wenger, S. Rentsch, et al., J. Power Sources 118, 1 (2003).
A. Mehranfar and M. Izadyar, RSC Adv. 5, 49159 (2015).
R. Kumar, A. Karkamkar, M. Bowden, et al., Chem. Soc. Rev. 48, 5350 (2019).
F. H. Stephens, V. Pons, and R. T. Baker, Dalton Trans. 36, 2613 (2007).
W. Chen, J. Ji, X. Duan, et al., Chem. Comm. 50, 2142 (2014).
Y. Ge, Z. H. Shah, X. Lin, et al., ACS Sustain. Chem. Eng. 5, 1675 (2017).
J. Kang, T. Chen, D. Zhang, et al., Nano Energy 23, 145 (2016).
S. Rej, C. F. Hsia, T. Y. Chen, et al., Angew. Chem. Int. Ed. 128, 7338 (2016).
H. Yen, Y. Seo, S. Kaliaguine, et al., ACS Catal. 5, 5505 (2015).
A. N. Kalenchuk, V. I. Bogdan, L. M. Kustov, et al., Fuel 280, 118625 (2020).
A. N. Kalenchuk, V. I. Bogdan, L. M. Kustov, et al., Fuel Process. Technol. 169, 94 (2018).
L. Ma, Q. Zhang, C. Wu, et al., Anal. Chim. Acta 1055, 17 (2019).
J. San Choi, C. W. Ahn, J. Bae, et al., Curr. Appl. Phys. 20, 102 (2020).
Q. Xu and M. Chandra, J. Alloys Compd. 446, 729 (2007).
F. Fu, C. Wang, Q. Wang, et al., J. Am. Chem. Soc. 140, 10034 (2018).
M. Chandra and Q. Xu, J. Power Sources 168, 135 (2007).
M. Li, J. Hu, Z. Chen, et al., RSC Adv. 4, 41152 (2014).
X. Yang, F. Cheng, J. Liang, et al., Int. J. Hydrogen Energy 36, 1984 (2011).
X. Yang, F. Cheng, Z. Tao, et al., J. Power Sources 196, 2785 (2011).
W. Fu, C. Han, D. Li, et al., J. Energy Chem. 41, 142 (2020).
C. Liu, Y. Wu, C. Chou, et al., Int. J. Hydrogen Energy 37, 2950 (2012).
U. B. Demirci and F. Garin, J. Alloys Compd. 463, 107 (2008).
ACKNOWLEDGMENTS
This work was supported by the Local Science and Technology Development Fund Projects Guided by the Central Government of China (no. 2021ZYD0060), the Science and Technology Project of Southwest Petroleum University (no. 2021JBGS03), and the Chengdu International Science and Technology Cooperation Fund (2020GH0200069HZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
DATA AVAILABILITY STATEMENT
The data that support this study are available in the article and accompanying online supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, H., He, J., Lei, B. et al. Highly Efficient Hydrogen Production from Ammonia Borane over Carbon Nanotubes Supported Pt–Ni Bimetallic Catalyst. Russ. J. Phys. Chem. 96, 1935–1943 (2022). https://doi.org/10.1134/S0036024422090357
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422090357