Abstract
SHS synthesis of titanium carbide and metalloceramics (cermets) based on it shows that transition from a powder mixture to a granular mixture reduces the effect of the release of impurity gas from component particles and ensures the stability of the mode of combustion without changing the phase composition of the target products. The combustion of 5Ti + 3Si powder and granular mixtures using titanium particles of different sizes is compared for the first time. The rate of combustion of a powder mixture of coarsely dispersed titanium is higher than that of finely dispersed titanium. According to the convective–conductive model of combustion, this is due to the effect of the release of impurity gas. The conditions formulated for heating the components of powder mixtures ahead of the combustion front explain the increase in the rate of combustion after mixture granulation for fine titanium and the drop for coarse titanium. In contrast to powder, the rate of combustion of a granular mixture of finely dispersed titanium is higher than that of coarsely dispersed titanium. For granular mixtures, where the effect impurity gases have on combustion is greatly reduced, the determining factor affecting the rate of combustion is thus the size of particles of the initial components. Varying the size of the granules from 0.6 to 1.7 mm, the authors use the experimental burning rate to calculate the rate of combustion of granule materials and the time of combustion transfer from granule to granule. The proposed criteria for localizing the release of impurity gas allow determination of whether granulation of a powder mixture is necessary when scaling the process of high-temperature synthesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The release of impurity gases is normally observed in SHS processes at temperatures of combustion on the order of several thousand degrees. The amount of such gases largely determines the mode of combustion. The heating of a charge containing titanium is accompanied by significant desorption of impurity gases [1–3]. Metal powders of different grades and even individual batches of the same grade can have different adsorption/desorption capacities of gasifying impurities [4]. Nonmetallic components can also make a substantial contribution to gas release, but the contribution from their crystalline modifications is low compared to their amorphous counterparts [3, 4]. The strong effect the release of impurity gases (IG) has on the velocity of a front in a powder medium is explained by the convective–conductive model of combustion (CCCM) [5, 6]. Granulation of the initial powder mixture, in which a change in the structure of the porous medium increases the gas permeability of filling by orders of magnitude, is used to stabilize the SHS processes. The products of synthesis obtained from the granular charge are granules of the same size that do not sinter with one another. The high gas permeability of the granulated charge does not change during combustion. Combined with the small size of the granules, this ensures the rapid removal of impurity gases from the reaction zone [7, 8].
When analyzing the literature on the combustion of a 5Ti + 3Si mixture, it is noteworthy that the main objects of study were pressed samples from a powder mixture [1, 9–15]. This system is considered a model for studying the process of so-called gasless combustion [1, 9], and the degassing of samples is often used in experiments. In [10], the synthesis of titanium silicides via thermal explosion using Ti particles of different sizes was found to be possible only after degassing the samples. Maznoi and Kirdyashkin [11] studied how a change in the size of Ti particles in a degassed 5Ti–3Si mixture affects the permeability of the products of synthesis. In other experimental works, samples were not preliminarily degassed. The possible effect of impurity gases was not considered in [12] when comparing the rate of combustion and temperature of samples of different stoichiometric compositions. In [13], where the measured temperatures and velocities of the combustion front of compact 5Ti + 3Si samples were used for numerically modeling the SHS kinetics, the heat balance equation considered only conductive heat transfer and convective and radiative losses, without the effects of gas. Qualitatively different dependences of the burning rate of a 5Ti–3Si mixture on the size of Ti particles were obtained in [14, 15], while the authors of both works considered the combustion of this mixture to be gasless. When interpreting the data in [15], the authors did not compare them to the results in [14].
The question of whether we must consider the effects of IG when interpreting experimental results and scaling the synthesis of titanium silicides therefore remains open.
The aim of this work was to identify the mechanism of the effect IG has on the combustion of a 5Ti + 3Si powder mixture, using granulation as a tool for eliminating the effect of the release of impurity gas. Another aim was to explain the nature of the change in combustion rate upon transition from powder to granular mixtures from the viewpoint of the convective–conductive model of combustion.
EXPERIMENTAL
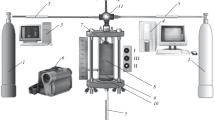
We studied patterns of combustion on an original experimental setup (Fig. 1). Considered mixture (8) was poured into a vertical transparent quartz tube (outer diameter, 19 mm; height, 90 mm; wall thickness, 2 mm) on mineral wool substrate (9) (Al2O3 base) (Fig. 2). Signals from sensors (5) and LEDs giving the position of the gas supply switch (11) were sent to the computer (4) in real time through an ADC. Combustion was initiated from the top end of the tube using a thermal pulse from tungsten spiral (7). Combustion was recorded by SONY FDR AX-700 camera (6) at 100–250 fps. The velocity of the combustion front was calculated via frame-by-frame processing of the video recordings. To avoid shrinkage of the unburned part of the filling during combustion and obtain consistent results, the samples were purged in an argon flow at a pressure drop of 1 atm before each experiment. The height of the filling of the initial mixture (powder and granular) after blowing was 40 ± 5 mm.
Scheme of the experimental setup: (1) nitrogen cylinder, (2) argon cylinder, (3) computer for recording video signal, (4) computer for recording sensor readings via ADC, (5) flow and pressure sensors, (6) digital video camera, (7) electric spiral, (8) mixture, (9) layer of mineral wool, (10) metal mesh, (11) gas switch (position I, nitrogen; II, argon; III, no gas).
The particle size distribution of the components was determined on a Microsizer-201 C laser analyzer. The phase composition of the final product was studied on a DRON-3M X-ray diffractometer using monochromatic CuKα radiation. Diffractograms were recorded by scanning in the 2θ = 20°–80° range of angles with a shooting step of 0.2°. The resulting data were analyzed using the PDF-2 database. The microstructure of titanium powders was studied via SEM on a Carl Zeiss Ultra Plus microscope.
Industrial Ti powders (Russia) of PTM grade with 99% purity of different fractional compositions were used to prepare the initial mixtures:
• fine (<54 μm), coarse (<169 μm);
• semiconductor Si powder (<2.1 μm, purity 99.99%);
• 4% polyvinyl butyral alcohol solution for granulation.
For brevity, we shall refer to the initial mixtures based on fine titanium mixtures as I, and those based on coarse titanium mixtures as II. Figure 2 shows SEM photos of the initial titanium powders.
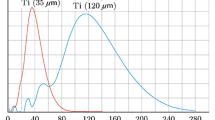
Figure 3 shows the mass size distribution of titanium particles of the initial powders.
The compositions were granulated by preliminarily stirring the initial powder mixtures for 4 h in a gravity mixer. A 4 wt % solution of polyvinyl butyral in ethanol was then added to the resulting mixture until a pasty mass formed that was rubbed through a sieve after mixing. The resulting particles were rolled on a rotating horizontal surface to make them spherical. They were then dried in air for 10 h and dispersed on a vibrating screen. Figure 4 shows photos of the initial powder and granulated 5Ti + 3Si mixtures with typical granule sizees D = 0.6 and 1.7 mm.
Granules of fractions were used in our experiments: 0.4–0.8, 0.8–1.2, 1.4–2 mm, and a wide fraction of 0.6–1.6 mm. In calculations, the granule size was taken as the average value of the size in each fraction: D = 0.6, 1, 1.7 mm and D = 1.1 mm, respectively.
RESULTS AND DISCUSSION
Figure 5 shows the frames of combustion of the 5Ti–3Si powder mixture and the granular mixture with granules D = 0.6 and 1.7 mm in size.
Figure 6 shows the dependence of the rates of combustion front propagation for granular compositions I and II on the size of the granules, along with the rates of combustion of powder mixtures I and II. The given rates of combustion are the average for 3–4 experiments, and the scatter of values is no more than 10%.
When analyzing the results shown in Fig. 6, note first that the rate of combustion of the powder mixture (line 3) for composition I based on finely dispersed titanium is lower than that of the granular mixture. At the same time, the rate of combustion for composition II with coarse-dispersed titanium slowed upon transition from the powder mixture to the granular mixture. In contrast to the powder mixture, the rate of combustion for all fractions of granular mixture I (line 1) was higher than that of mixture II (curve 4). The rate of combustion of mixture I (U = 29 mm/s) did not change when average granule size D was raised from 0.6 to 1.7 mm, whereas our experiments showed an increase in the velocity of the combustion front (from 14.5 to 20 mm/s) for mixture II.
According to XRD data, the phase composition of the products of combustion for granular mixtures I and II did not change when the granule size was raised from 0.6 to 1.7 mm. For the powder and granular mixtures, the X‑ray patterns contained only the peaks of the Ti5Si3 phase (Fig. 7).
The rate of combustion thus increased for mixture I based on finely dispersed Ti and decreased for mixture II with coarsely dispersed Ti upon the transition from powder to granular mixtures, despite the identical composition of the initial mixture and the phase composition of the products of combustion. The theory of flame front propagation in a heterogeneous condensed medium [16] based on a conductive mechanism of heat transfer in the combustion wave, cannot explain the difference between the change in the rate of combustion after granulation for mixtures I, II.
We must turn to the convective–conductive model of combustion (CCCM) to explain these results [5, 6]. In this model, the velocity of the movement of the melt layer under the action of capillary forces and the pressure difference of the impurity gases in front of and behind the melt layer is the apparent velocity of the combustion front. According to CCCM, the increased pressure of impurity gases in a powder mixture ahead of the reaction front (melt layer) slows the rate of combustion. The increased pressure behind the front accelerates it. We must therefore determine whether the particles of the initial components have time to warm up and release some of the impurity gases ahead of the combustion front.
Let us assume that in the powder mixture, the particles of the initial components have time to warm up ahead of the combustion front when two conditions are met simultaneously. First, characteristic particle size d must be less than the width of the zone of heating: L = a/Up, where Up is the experimental burn rate of the powder mixture, and a is its thermal diffusivity:
Second, a particle’s time of thermal relaxation th = d2/(4a1) (where a1 is the thermal diffusivity of the particle’s material) is less than its characteristic time spent in the warming up zone of the combustion wave, t = L/Up = a/(Up)2 [1]:
Table 1 shows the results from calculating th for Ti particles of different sizes, along with L and t for mixtures I, II.
The values of maximum size distribution were taken as the characteristic sizes d of Ti particles: d = 35 μm for fine and d = 120 μm for coarse titanium (Fig. 3). The parameter values below were used in our calculations: a = 10−6 m2/s [17], a1(Ti) = 8 × 10−6 m2/s [18], a1(Si) = 5 × 10−5 m2/s [19]. For Si particles with characteristic sizes of around 2 μm, the value of th = 2 × 10−8 s is orders of magnitude lower than that of titanium, and heating conditions (1), (2) are met. The IG from Si (if present) is therefore ahead of the combustion front for both powder mixtures and should have the same effect on the rate of propagation of the combustion front inside them. However, the IG from silicon particles is at the level of Ti + C samples after thermal vacuum treatment [3], and thus has no appreciable effect on the rate of front propagation.
Calculated results show that condition (2) is satisfied for the titanium powders used in our experiments. Heating condition (1) is satisfied only for titanium with d = 35 μm (mixture I). The IG from titanium is thus in the zone of heating of the combustion wave as powder mixture I burns and slows its propagation, in contrast to mixture II with d = 120 μm. Burn rate Up of the powder mixture is therefore lower for mixture I than for mixture II (Fig. 6).
According to the widely used theory in [16], the reduction of the particles of initial reagents should raise the rate of combustion. Experimental results for powder mixtures contradict the conclusions of the work [16] but correspond to them for granular mixtures.
To explain why the rate of combustion rises for mixture I and falls for mixture II upon transition from powder to granular (Fig. 6), we must consider features of combustion front propagation in granular systems. A granulated mixture consists of individual cells (granules) that contain mixed reagents and are capable of self-combustion, and the pore space between them occupied by gas. It is because of the discreteness of granular mixtures and the difference between the sizes of granules and grains of the resulting product that surface tension forces prevent the melt from flowing beyond individual granules. The high gas permeability of such a charge therefore does not change during combustion. The rate of combustion of a granular mixture is determined by both the rate of combustion of individual granules and the rate of heat transfer from granule to granule, which depends on the contact area and the efficiency of conductive heat exchange between granules. Since the granules are much larger than the initial components used in our experiment, the combustion of an individual granule can be considered similar to that of a powder mixture. However, better conditions are created in the granule for removing IG from the combustion zone than with powder filling, since the length of the filtration zone along a granule to the porous space between granules is no more than half its diameter. Combined with the high gas permeability of the filling, this has a negligible IG effect on both the combustion of the granules themselves and the sample as a whole [8]. If there is no IG in the zone of heating in the powder mixture, granulation slows the burning of the sample, due to the stage of combustion transfer from granule to granule. Such conditions are created in mixture II with coarsely dispersed titanium. In contrast, granulation raises the rate of combustion for a composition where impurity gases are released in the zone of heating and have a retarding effect. The combustion of powder mixture II with finely dispersed titanium meets these conditions.
It was of independent interest to study the parameters of combustion of physically isolated cells of a dispersed mixture (individual granules). The granules of mixtures I and II kept their sizes during combustion and did not sinter with one another, so we may assume that heat is transferred between them mainly at the points of contact between the granules and is determined by the conductive heat transfer mechanism [20]. As in [21], we can calculate such characteristics as rate \({{{v}}_{{{\text{com}}}}}\) of combustion of the granule material and time tig of combustion transfer from granule to granule. A necessary condition for applying this approach is meeting the inequality
where D is the size of the granule and h is the depth of heating of the granule at the time of ignition;
where a is the thermal diffusivity of the granule, 10‒6 m2/s [17]; and
where tb is the time of the granule combustion, calculated from the experimental data. Using the experimental rates of combustion (Fig. 6) and formulas (4), (5) we obtain (upper estimate) that depth h of granule heating grows from 0.14 to 0.24 mm for mixture I and from 0.2 to 0.29 mm for mixture II when the size of granules changes from 0.6 mm to 1.7 mm. Since h < D for granules of all sizes, their heating up to the moment of ignition can be described by the model of a semi-infinite body. The time of the transfer of combustion from one granule to another can therefore be considered equal for granules of different sizes.
In the conductive mode, the time tb of granule combustion (5) in a filling of a large number of granules is equal to the sum of the time tcom of the combustion of granule material and tig of the time of combustion transfer from granule to granule:
Assuming that rate \({{{v}}_{{{\text{com}}}}}\) of the combustion of the mixture’s material is the same for granules of different sizes, we can calculate \({{{v}}_{{{\text{com}}}}}\) and tig.
After substituting (5) into formula (6) and replacing tcom = D/\({{{v}}_{{{\text{com}}}}}\), we obtain an expression associating the experimental value of the granular mixture rate of combustion U in the conductive mode with \({{{v}}_{{{\text{com}}}}}\) and tig:
Substituting into (7) values D and U for two fractions of granules, we obtain a system of two equations with two unknowns, the solving of which yields values \({{{v}}_{{{\text{com}}}}}\) and tig. Substituting obtained values \({{{v}}_{{{\text{com}}}}}\) and tig into (7), we can calculate the burning rate of a mixture of granules of a different size.
As noted above, the burning rate of the mixture I did not depend on the size of the granules: U = 29 mm/s. We can see from expression (6) that the burning rate for granules of different sizes is the same, so long as tig \( \ll \) tcom. For granular mixture I, the velocity of front propagation is virtually equal to velocity U = \({{{v}}_{{{\text{com}}}}}\) of combustion of the granule material. Note that \({{{v}}_{{{\text{com}}}}}\) = 29 mm/s is notably higher than the rate of combustion of the initial powder mixture, Up = 21 mm/s.
For mixture II, the solution to the equations obtained from (7) when substituting the data for the granule fractions with D = 0.6 and 1.7 mm yields tig = 0.0176 s, \({{{v}}_{{{\text{com}}}}}\) = 25.2 mm/s. To confirm these data, we use the obtained values of tig and \({{{v}}_{{{\text{com}}}}}\) to calculate the rate of combustion of the medium fraction with D = 1 mm. We obtain 18 mm/s, which is virtually the same as the average experimental value U = 17.5 mm/s. Similar calculations of the rates of combustion for fractions with D = 0.6 and 1 mm yield tig = 0.0177 s and \({{{v}}_{{{\text{com}}}}}\) = 25.4 mm/s. The rate of combustion of a granular mixture with D = 1.7 mm calculated from these data is U = 20 mm/s, which coincides with the average value obtained in our experiments. We can see that the values of \({{{v}}_{{{\text{com}}}}}\) and tig, calculated from the experimental data for different granules practically coincide. Note that for mixture II, \({{{v}}_{{{\text{com}}}}}\) = 25.3 mm/s virtually coincides with rate of combustion Up = 25 mm/s of the initial powder mixture. Calculations confirm the correctness of assuming the conductive mode of combustion of granular mixture II for all given fractions of granules: the time of the granule ignition does not depend on their size, and the rate of combustion of the granule material is the same for all fractions.
According to our experimental results, period tcom of the combustion of an individual granule rose from 0.021 to 0.051 s for mixture I and from 0.024 to 0.067 s for mixture II when the size of granules was changed from 0.6 to 1.7 mm. In contrast to granular Ti + C mixtures [21], the period of combustion of the granule material was longer than the maximum period tig ≈ 0.0177 of combustion transfer from granule to granule for granules of all fractions.
CONCLUSIONS
Our study showed that the 5Ti + 3Si powder mixture cannot be considered gasless, since only with the convective–conductive model of combustion were we able to explain the unusual (from the viewpoint of the classical gas-freeless combustion theory [16]) dependence of the rate of combustion on the sizes of the component particles and the change in the burn rate upon transition from powder to granular mixtures. The criteria for the area of IG localization formulated for the first time in this work allowed us to determine whether the effect impurity gases have on the mode of combustion of a powder mixture is significant, and therefore to decide logically whether there is a need for granulation of the initial powder mixture when scaling the process of high-temperature synthesis.
1. The reason for the higher burning rate of the 5Ti–3Si powder mixture with coarse titanium powder versus finely dispersed titanium powder was established.
2. The rate of combustion of a granule’s material and the time of combustion transfer from granule to granule for mixtures with titanium of different fractions were calculated using experimental data of combustion rates.
3. The mechanism of changes in the rate of combustion upon transition from powder to granular mixtures was explained from the viewpoint of the convective–conductive model of combustion.
4. Necessary and sufficient conditions for heating the components of powder mixtures in the combustion wave zone of heating were formulated and confirmed experimentally for the first time.
5. It was found that the effect the size of a titanium particle has on the rate of combustion for both powder and granular mixtures is not related to the difference between the phase compositions of condensed products and the completeness of the conversion of the initial reagents.
REFERENCES
A. S. Rogachev and A. S. Mukasyan, Combustion for Material Synthesis (CRC, Taylor and Francis Group, New York, 2015), p. 398.
A. S. Mukas’yan, V. A. Shugaev, and R. M. Kirtyakov, Fiz. Goreniya Vzryva 2 (1), 9 (1993). https://doi.org/10.1007/BF00755319
A. G. Merzhanov, A. S. Rogachev, L. M. Umarov, and N. V. Kir’yakov, Fiz. Goreniya Vzryva 33 (4), 55 (1997).
V. I. Vershinnikov and A. K. Filonenko, Fiz. Goreniya Vzryva 14 (5), 42 (1978). https://doi.org/10.1007/BF00789716
B. S. Seplyarskii, Dokl. Phys. Chem. 396, 130 (2004). https://doi.org/10.1023/B:DOPC.0000033505.34075.0a
N. M. Rubtsov, B. S. Seplyarskii, and M. I. Alymov, Ignition and Wave Processes in Combustion of Solids (Springer Int., Cham, Switzerland, 2017), Chap. 4, p. 117. https://doi.org/10.1007/978-3-319-56508-8_4
A. P. Amosov, A. G. Makarenko, A. R. Samboruk, et al., Int. J. Self-Propag. High-Temp. Synth. 19, 70 (2010). https://doi.org/10.3103/S10613862100101274
B. S. Seplyarskii and R. A. Kochetkov, Int. J. Self-Propag. High-Temp. Synth. 26, 134 (2017). https://doi.org/10.3103/S106138621702011X
S. G. Vadchenko, Fiz. Goreniya Vzryva 38 (1), 55 (2002). https://doi.org/10.1023/A:101400590093
J. Trambukis and Z. A. Munir, J. Am. Ceram. Soc. 73, 1240 (1990). https://doi.org/10.1111/j.1151-2916.1990.tb05186.x
A. S. Maznoi and A. I. Kirdyashkin, Combust. Explos., Shock Waves 50, 60 (2014). https://doi.org/10.1134/S0010508214010079
C. L. Yeh, H. J. Wang, and W. H. Chen, J. Alloys Compd. 450, 200 (2008). https://doi.org/10.1016/j.jallcom.2006.10.074
C. L. Yeh, P. W. Hwang, and Y. L. Chen, J. Alloys Compd. 714, 567 (2017). https://doi.org/10.1016/j.jallcom.2017.04.283
A. R. Sarkisyan, S. K. Dolukhanyan, I. P. Borovinskaya, and A. G. Merzhanov, Fiz. Goreniya Vzryva 14 (3), 49 (1978).https://doi.org/10.1007/BF00740494
A. S. Rogachev and A. S. Mukas’yan, Combust. Explos., Shock Waves 51, 53 (2015). https://doi.org/10.1134/S0010508215010050
A. P. Aldushin, T. M. Martem’yanova, A. G. Merzhanov, et al., Fiz. Goreniya Vzryva 8 (2), 202 (1972). https://doi.org/10.1007/BF00740444
A. A. Zenin, A. G. Merzhanov, and G. A. Nersisyan, Combust. Explos. Shock Waves 17, 63 (1981). https://doi.org/10.1007/BF007727879
T. Slezak, J. Zmywaczyk, and P. Koniorczyk, AIP Conf. Proc. 2170, 020019 (2019). https://doi.org/10.1063/1.5132738
B. Abeles, D. S. Beers, G. D. Cody, and J. P. Dismukes, Phys. Rev. 125, 44 (1962).
B. S. Seplyarskii, R. A. Kochetkov, and S. G. Vadchenko, Combust. Explos., Shock Waves 52, 665 (2016). https://doi.org/10.1134/S001050821606006X
B. S. Seplyarskii, R. A. Kochetkov, T. G. Lisina, and N. I. Abzalov, Combust. Explos., Shock Waves 57, 60 (2021). https://doi.org/10.1134/S001050822101007X
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interest.
Additional information
Translated by V. Selikhanovich
Rights and permissions
About this article
Cite this article
Seplyarskii, B.S., Kochetkov, R.A., Lisina, T.G. et al. Macrokinetic Mechanism of the Combustion of 5Ti + 3Si Powder and Granular Mixtures: Effects of the Release of Impurity Gas and the Size of Titanium Particles. Russ. J. Phys. Chem. 96, 977–984 (2022). https://doi.org/10.1134/S0036024422050260
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024422050260