Abstract
The effect redox treatment has on the stability of supported heteropoly compounds with Keggin structure in the KxH6 – х [SiW11NiO39] + PtCl4/ZrO2–Al2O3, KxH6 – х[SiW11NiO39] + PtCl4/Al2O3, and KxH6 – х[SiW11CoO39] + H2PtCl6/ZrO2–Al2O3 hybrid catalysts is studied via DRIFTS using CO as a probe molecule. It is found that the electronic state of nickel and cobalt remains unchanged during the redox treatment under the static conditions at 300°C. The Ni-HPC and Co-HPC complexes are stable during redox treatment. Platinum is in three electronic states in the calcined Ni-HPC + PtCl4/ZrO2–Al2O3 sample: Pt4+, Pt2+, and Pt+. Redox treatment reduces platinum to Pt+ cations. Platinum is in two electronic states in the calcined Ni-HPC+PtCl4/Al2O3 sample: Pt2+ and Pt+. Redox treatment reduces platinum to Pt+ cations and small metal particles. A comparison of two Ni-HPC + PtCl4 samples supported on different carriers indicates that ZrO2 in the carriers prevents the reduction of platinum to metal. Platinum is in two electronic states in the Co-HPC + H2PtCl6/ZrO2–Al2O3 calcined sample: Pt4+ and Pt+. Redox treatment reduced platinum to form Pt+.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Individual and supported catalysts based on heteropoly compounds (HPCs) with Group VIII metals in the internal coordination sphere have been studied since the 1950s. They are effective catalysts of acid and redox reactions [1–11]. The salts of heteropoly acids are mainly used in acid catalysis. HPCs attributed to the 12th series of a Keggin structure are the ones most common and important for catalysis. These structures remain unchanged upon the substitution of heteroatoms in the outer sphere, hydration-dehydration, dissolution, and under mild redox conditions. The synthesis of supported catalytic systems in which mixed heteropoly complexes containing Co, Ni, Pt, and any other transition metals in the inner coordination sphere (partially replacing W or Mo in the conventional Keggin structure) are used as active components, is a fundamentally new approach to obtaining highly dispersed metal particles on a support’s surface. High dispersion of Pt and Pd in HPA/Al2O3 supported systems (or preparations of atomically dispersed metal centers) was observed in [12, 13].
Supported systems with active highly dispersed metal centers can be used in a number of processes. The isomerization of n-paraffins in particular proceeds on bifunctional catalysts containing acid and metal sites on the surface. The process occurs in the presence of hydrogen; i.e., the catalyst is in a reductive environment. At the same time, the catalytic afterburning (e.g., of exhaust gases from automobiles or industrial objects on highly dispersed metal catalysts of the applied type) proceeds under inherent oxidative conditions.
The aim of this work was to study supported Ni-HPC and Co-HPC systems and the state of Pt in hybrid catalysts, and to determine the resistance of metal centers to sintering during prolonged operation under oxidative and reductive conditions.
EXPERIMENTAL
Diffuse reflectance IR (DRIFTS) spectra were obtained at room temperature in the range of 6000–400 cm–1 with a step of 4 cm–1 on a NICOLET Protege 460 spectrometer equipped with a diffuse reflection accessory [14]. Five hundred spectra were collected to obtain a satisfactory signal-to-noise ratio. CaF2 powder was used as a standard. The catalyst samples (0.25 mm fraction) were placed into tubes before IR measurements and subjected to vacuum treatment for 2 h at 400°C (heating rate, 5°C/min) to remove physically adsorbed water. The tubes with the samples were then cooled to room temperature, CO was adsorbed at an equilibrium pressure of 15 Torr, and the spectra were recorded. CO was subsequently desorbed in vacuum. The spectra were recorded after each desorption temperature. Adsorption–desorption procedures were performed in the same way after each successive treatment of a sample in the same tube. The samples were reduced with H2 for 1 h at 20 Torr and 300°C. Hydrogen was replaced by evacuation several times during this treatment to remove the adsorbed water formed during reduction.
The intensity of absorption bands was expressed in Kubelka–Munk units. The data were obtained and processed using the OMNIC software. The spectra of adsorbed CO were taken as the difference between those recorded before and after the adsorption of test molecules.
Mixed heteropoly compounds (HPCs) with Keggin structure containing Ni and Co heteroatoms, which occupied the position of a tungsten atom in a W3O13 fragment [9], were prepared according to the standard procedure [1, 13]. Our samples were prepared by impregnating ZrO2–Al2O3 double oxide or Al2O3 with aqueous solutions of the corresponding HPCs [12, 13]. IK-GO-1 Al2O3 (230 m2/g) and ZrO2–Al2O3 (85 m2/g) containing Al2O3 in amounts of ~15 wt % were used as carriers. The amounts of Ni and Co in the supported HPC samples were ~0.5 wt %. HPCs were dried and calcined at 400°C in an air flow.
Three deposited HPC samples were studied in this work: KxH6−х[SiW11NiO39] + PtCl4/ZrO2–Al2O3 was a hybrid sample containing two salts (HPC with nickel in the inner coordination sphere of HPA and platinum chloride). KxH6−х[SiW11NiO39] + PtCl4/Al2O3 was the same as the other hybrid sample, but it was deposited on aluminum oxide. KxH6−х[SiW11CoO39] + H2PtCl6/ZrO2–Al2O3 is a hybrid sample containing two salts (HPC with cobalt in the inner coordination sphere of HPA and chloroplatinic acid). Platinum chloride supported on a double carrier (PtCl4/ZrO2–Al2O3) was also studied for comparison. The amount of Pt in all the samples was ~0.5 wt %.
RESULTS AND DISCUSSION
Figure 1 shows the IR spectra obtained during the adsorption–desorption of CO on a PtCl4/ZrO2–Al2O3 sample that underwent subsequent redox treatment. Two bands of adsorbed CO were observed in the spectrum of this sample after calcination at 400°C (Fig. 1a): a narrow one at 2187 cm−1 and an asymmetric one that has a center at 2126 cm−1 and a shoulder at lower frequencies. Reduction in H2 (Fig. 1b) at 300°C caused the low-frequency tail of the band at 2126 cm−1 to disappear. Two new bands appeared in the spectrum (Fig. 1c) at 2090 and 1851 cm−1 after subsequent reoxidation at 300°C. These two bands then disappeared after the next regeneration (Fig. 1d). The final reoxidation did not produce any changes in the spectrum (Fig. 1e). The band at 2187 cm−1 was in the same position after all treatments.
DRIFT-spectra of CO adsorption–desorption on PtCl4/ZrO2–Al2O3 sample after different treatments (α is absorption and k is the wavenumber) in (a) air at 400°C, (b) air at 400°C and H2 at 300°C, (c) air at 400°C, H2 at 300°C, and air at 300°C, (d) air at 400°C, H2 at 300°C, air at 300°C, and H2 at 300°C, and (e) air at 400°C, H2 at 300°C, air at 300°C, H2 at 300°C, and air at 300°C.
The band at 2187 cm−1 is probably a superposition of those from linear carbonyls at the Lewis acid sites (LASes) of the carrier [15, 16], [Pt4+(CO)Cl5]– ionic carbonyl after the decomposition of platinum chloride [17], and linear carbonyl on Pt4+ cations [16, 18–23]. The band at 2126 cm−1 can be attributed to the valence vibrations of C≡O bonds in carbon monoxide molecules adsorbed linearly on Pt2+ cations; the band at 2090 cm−1, to ones adsorbed on platinum metal particles. The band at 1851 cm–1 can be attributed to a bridging adsorption form of CO on Pt0 [18–23].
The bands of linear carbonyls on coordination unsaturated carrier cations (LASes), including platinum cations, were the first to disappear from the IR-CO spectra of the PtCl4/ZrO2–Al2O3 sample during CO desorption in vacuum, regardless of preliminary redox treatments. The desorption of CO in vacuum at room temperature led to the disappearance of the carbonyl band on LASes in the spectrum of initial calcined sample (Fig. 1a). The band of Pt4+–CO carbonyl at 2177 cm–1 and that of Pt2+–CO at 2126 cm−1 with the shoulder at 2079 cm−1 of carbonyl on platinum metal remained in the spectrum. Raising the temperature of desorption to 150°С changed the spectrum: the bands at 2177 cm−1 disappeared, the intensity of the band at 2126 cm−1 was reduced, the shoulder at 2108 cm−1 of a Pt+–CO carbonyl became clear, and the band at 2066 cm−1 attributed to linear carbonyl on platinum metal appeared. Raising the temperature of desorption to 300°С resulted in two bands with similar intensities at 2093 and 2057 cm−1 remaining in the spectrum. Both bands can be attributed to linear monocarbonyls on Pt metal particles of different dispersities. Reduction of the initial calcined sample produced a similar spectral pattern (Fig. 1b). The positions of singletons on particles of metallic platinum remained almost unchanged (2092 and 2051 cm−1). Subsequent oxidation resulted in bands of linear and bridging adsorption forms on metallic platinum being observed in the spectrum at 2090, 2051, and 1851 cm−1 in the presence of CO (Fig. 1c). Further reduction did not produce a band of bridging carbonyls in the spectrum (Fig. 1d), and the singletons on metallic platinum retained their positions at 2093 and 2053 cm−1. Final oxidation produced the same spectral pattern observed in the presence of CO on the calcined sample (Fig. 1e). Thermal desorption at 300°C resulted in the broad band at around 2093 cm−1 in the spectrum.
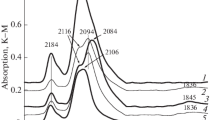
Figure 2 shows IR spectra recorded during the adsorption–desorption of CO on the KxH6−х[SiW11NiO39] + PtCl4/ZrO2–Al2O3 sample subjected to successive redox treatment. Three intense bands are clearly seen at 2199, 2150, and 2098 cm−1, along with the one weak band at 2364 cm–1 from adsorbed CO in the spectrum of this sample after calcination at 400°C (Fig. 2a). The reduction of Н2 at 300°C, subsequent reoxidation, subsequent re-reduction, and final reoxidation at the same temperature did not change the positions of these lines in the spectrum (Figs. 2b–2e). Only the ratio of their intensities changed.
DRIFT spectra of CO adsorption–desorption on KxH6−х [SiW11NiO39] + PtCl4/ZrO2–Al2O3 sample after different treatments. Denotations are the same as in Fig. 1.
The band at 2364 cm−1 can be attributed to valence vibrations of C=O bonds in CO2 molecules that form during the oxidation of CO on Ni2+ cations [15, 16]. The band at 2199 cm−1 was probably due to linear complexes of CO with the LASes of the carrier [15, 16], [Pt4+(CO)Cl5]– ionic carbonyl remaining after the decomposition of platinum chloride [17], and linear carbonyl on Pt4+ cations [16, 18–23]. The band at 2150 cm−1 can be attributed to the valence vibrations of C≡O bonds in carbon monoxide molecules adsorbed linearly on Pt2+ cations; the band at 2098 cm−1, to ones adsorbed on Pt+ cations and/or large clusters of metallic platinum [15–23].
The bands of adsorbed СО2 and linear carbonyls on coordination unsaturated carrier cations (LASes) were the first to disappear from the IR-CO spectra of the KxH6 – х[SiW11NiO39] + PtCl4/ZrO2–Al2O3 sample during desorption in vacuum, irrespective of preliminary treatments. The desorption of CO in vacuum at 20 and 150°C (Fig. 2a) reduced the intensity of the 2199 cm−1 band and shifted it 5 cm−1 toward lower frequencies. It also reduced the intensity of the band at 2150 cm−1, and shifted the one at 2098 cm−1 to 2089 cm−1. Raising the temperature of desorption to 300°С resulted in one band remaining in the spectrum at 2150 cm−1 with a clear tail at lower frequencies. This band can be attributed to linear monocarbonyl on Pt2+ cations, while the tail indicates there were more reduced cations and Pt metal particles with different dispersions. Reduction of the initial calcined sample and the subsequent oxidation–reduction–oxidation cycle produced a similar spectral pattern (Figs. 2b–2d). A slight shift of the band at 2104–2098 cm−1 during the desorption of CO in vacuum indicated it can be attributed to Pt+–CO linear carbonyls.
The next sample contained just another carrier than in the previous one, but the spectral pattern for these two samples differed considerably. Figure 3 shows the IR spectra recorded during the adsorption–desorption of CO on the KxH6−х[SiW11NiO39] + PtCl4/Al2O3 sample that underwent subsequent redox treatment. Two bands of adsorbed CO were observed in the spectrum of this sample after calcination at 400°C (Fig. 3a): a wide one at 2089 cm−1 with a shoulder at 2126 cm−1 and a narrow one at 2202 cm−1. Reduction in H2 at 300°C led to splitting of the broad band into two with maxima at 2126 and 2104 cm−1 (Fig. 3b). These three bands were observed in the spectrum after subsequent reoxidation, but the band at 2104 cm−1 appeared as a shoulder (Fig. 3c). The intensity of the band at 2126 cm−1 fell, while the other shifted to 2089 cm–1 after subsequent regeneration (Fig. 3d). Final reoxidation had the opposite effect (Fig. 3e). The band at 2202 cm−1 did not change its position after any treatment.
DRIFT spectra of CO adsorption–desorption on KxH6−х [SiW11NiO39] + PtCl4/Al2O3 sample after different treatments. Denotations are the same as in Fig. 1.
The bands at 2126 and 2104 cm−1 can be attributed to the valence vibration of C≡O bonds in carbon monoxide molecules linearly adsorbed on Pt2+ and Pt+ cations, respectively, while the band at 2098 cm−1 can be ascribed to metallic platinum particles [15–23]. The band at 2202 cm–1 seems to be attributable to the linear complexes of CO with LASes of the carrier [15, 16], the [Pt4+(CO)Cl5]– ionic carbonyl that remains after the decomposition of platinum chloride [17], and linear carbonyl on Pt4+ cations [15, 16, 18–23].
The bands of linear carbonyls on coordination unsaturated carrier cations (LASes), including those on platinum cations, were the first to disappear from the IR-CO spectra of the KxH6 – х[SiW11NiO39] + PtCl4/Al2O3 sample during the CO desorption in vacuum, irrespective of preliminary treatments. The bands at 2086 and 2076 cm–1 remained in the spectrum of the calcined sample (Fig. 3a) after desorption at 20 and 150°C, respectively. Raising the temperature of desorption to 300°C leads to two bands at 2097 and 2057 cm–1 with similar intensities. Both bands can be attributed to linear monocarbonyls on Pt metal particles with different dispersities. Subsequent reduction at 300°C generally reduced the intensity of lines in the spectrum, due probably to the destruction of the HPC structure. The relative intensity of the band at 2126 cm−1 grew, and the shoulder at 2108 cm−1 becomes clear. The bands at 2096 and 2079 cm−1 remained in the spectrum of the reduced sample after desorption at 20 and 150°C, respectively (Fig. 3b). Subsequent oxidation produced a spectral pattern similar to the one observed for the previous treatment (Fig. 3c).
The bands at 2094 and 2076 cm–1 remained in the spectrum of the oxidized sample after desorption at 20 and 150°C. Raising the temperature of desorption to 300°C produced a symmetric band at 2117 cm–1. Further reduction resulted in the band of carbonyl on LASes of the carrier disappearing and a change in ratio of the intensities of the bands at 2122 and 2089 cm−1 (Fig. 3d). The bands at 2083 and 2068 cm–1 remained in the spectrum of the re-reduced sample after the desorption at 20 and 150°C. Final oxidation resulted in the band of carbonyl on the LASes of the carrier at 2204 cm−1 appearing in the spectrum. The bands at 2094 and 2076 cm−1 remained in the spectrum of the reoxidized sample after desorption at 20 and 150°C (Fig. 3e). Raising the temperature of desorption to 300°C leads to emergence of a band at 2116 cm–1 with a shoulder at 2061 cm–1.
Figure 4 shows IR spectra recorded during the adsorption–desorption of CO on the KxH6−x[SiW11CoO39] + H2PtCl6/ZrO2–Al2O3 sample that underwent subsequent redox treatments. Three narrow bands of adsorbed CO are clearly seen in the spectrum of this sample after calcination at 400°C (Fig. 4a): at 2358, 2204, and 2103 cm−1. Reduction in H2 at 300°C (Fig. 4b) raised the intensity of the band at 2358 cm−1. Further reoxidation at 300°C, re-reduction, and final reoxidation at the same temperature did not produce any changes in the positions of the lines (Figs. 4c–4e).
DRIFT-spectra of CO adsorption–desorption on KxH6−х[SiW11CoO39] + H2PtCl6/ZrO2–Al2O3 sample after different treatments. Denotations are the same as in Fig. 1.
The band at 2358 cm−1 can be attributed to the valence vibrations of a C=O bond in CO2 molecules that form during the oxidation of CO on the Co2+ cations [15, 16]. The band at 2204 cm−1 was probably due to linear complexes of CO with the LASes of the carrier [15, 16], [Pt4+(CO)Cl5]– ionic carbonyl remaining after the decomposition of platinum chloride [17], and linear carbonyl on Pt4+ cations [15, 16, 18–23]. The band at 2103 cm–1 can be ascribed to the valence vibrations of C≡O bonds in carbon monoxide molecules linearly adsorbed on either Pt+ cations or fairly large particles of metallic platinum [15–23]. The weak band at 1882 cm−1 observed only in the spectrum of the initial calcined sample can be attributed to the bridging form of CO adsorption on metallic platinum.
The bands of adsorbed СО2 and linear carbonyls on coordination unsaturated carrier cations (LASes), including platinum cations and bridging carbonyls on platinum metal particles, were the first to disappear from the IR-CO spectra of the KxH6 – х[SiW11CoO39] + H2PtCl6/ZrO2–Al2O3 sample during CO desorption in vacuum, irrespective of preliminary treatments. The spectrum of the calcined sample (Fig. 4a) displayed bands at 2357 cm−1 (weak), 2204 cm−1, a shoulder at 2156 cm−1, an intense band at 2103 cm−1, and a weak band at 1882 cm−1. The bands at 2101 and 2091 cm−1 remained in the spectrum after desorption at 20 and 150°С, respectively. Raising the temperature of desorption to 300°C resulted in all bands disappearing. Further reduction at 300°C led to a general increase in the intensity of lines in the spectrum. The bands at 2099 and 2091 cm−1 remained in the spectrum of the reduced sample due to desorption at 20 and 150°C, respectively (Fig. 4b). Raising the temperature of desorption to 300°C caused all bands to disappear. Further oxidation produced a spectral pattern similar to the one observed for the previous treatment (Fig. 4c). The bands at 2099 and 2089 cm−1 remained in the spectrum of the reoxidized sample after desorption at 20 and 150°C. Raising the temperature of desorption to 300°C resulted in all bands disappearing. Further reduction produced a spectral pattern similar to the one obtained for the previous treatment (Fig. 4d). The bands at 2098 and 2089 cm−1 remained in the spectrum of the re-reduced sample after desorption at 20 and 150°C. Final oxidation produced a spectral pattern similar to the one recorded for the previous treatment (Fig. 4e). The bands at 2100 and 2089 cm−1 remained in the spectrum of the reoxidized sample after desorption at 20 and 150°C. Raising the temperature of desorption to 300°C resulted in all bands disappearing. A slight shift of the band at 2105–2089 cm−1 during CO desorption in vacuum indicated it can be attributed to Pt+–CO linear carbonyls.
CONCLUSIONS
We studied heteropolyacid catalysts containing Ni and Co heteroatoms that partially replace the W in Keggin structures of the 12th row with PtCl4 or H2PtCl6 as additives and supported on ZrO2–Al2O3 or Al2O3. Our findings are given below.
1. Platinum was in two electronic states in the initial calcined PtCl4/ZrO2–Al2O3 sample: Pt4+ and Pt2+. Multiple treatments of the calcined sample in H2 and in air at 300°C led to the reduction of platinum to form Pt+ cations and small metal particles.
2. Platinum was in three electronic states in the initial calcined KxH6 – х[SiW11NiO39] + PtCl4/ZrO2–Al2O3 sample: Pt4+, Pt2+, and Pt+. Multiple treatments of the calcined sample in H2 and in air at 300°C led to the reduction of platinum to form Pt+ cations.
3. Platinum was in two electronic states in the initial calcined KxH6 – х[SiW11NiO39] + PtCl4/Al2O3 sample: Pt2+ and Pt+. Multiple treatments of the calcined sample in H2 and in air at 300°C led to the reduction of platinum to Pt+ cations and small metal particles.
4. Platinum was in two electronic states in the initial calcined KxH6 – х[SiW11CoO39] + Н2PtCl6/ZrO2–Al2O3 sample: Pt4+ and Pt+. Multiple treatments of the calcined sample in H2 and in air at 300°C led to the reduction of platinum to Pt+ cations.
5. The electronic state of nickel and cobalt during redox treatments and under static conditions at 300°C remained unchanged. Ni-HPC and Co-HPC complexes were resistant to redox treatments.
6. A comparison of two KxH6 – х[SiW11NiO39] + PtCl4/ZrO2–Al2O3 and KxH6 – х[SiW11NiO39] + PtCl4/Al2O3 samples supported on different carriers showed that ZrO2 in the carrier prevents the reduction of platinum to metal, so the degree of platinum reduction depends on the acidic properties of the carrier. This result agrees with data obtained earlier for Pt-containing catalysts supported on carriers with different acidic properties (ZrO2, ZrO2–Al2O3, and Al2O3).
REFERENCES
C. Tourne, C. R. Acad. Sci. Ser. C 266, 702 (1968).
S. Suzuki, K. Kogai, and Y. Ono, Chem. Lett. 13, 699 (1984).
Y. Ono, M. Taguchi, S. Gerile, S. Suzuki, and T. Baba, Stud. Surf. Sci. Catal. 20, 167 (1985).
M. Misono, Catal. Rev.-Sci. Eng. 29, 269 (1987).
I. V. Kozhevnikov, Russ. Chem. Rev. 56, 811 (1987).
K. Na, T. Okuhara and M. Misono, Chem. Lett. 22, 1141 (1993).
K. Na, T. Okuhara, N. Mizuno, and M. Misono, Adv. Catal. 41, 113 (1996).
K. Na, T. Okuhara and M. Misono, J. Catal. 170, 96 (1997).
M. Misono, Chem. Commun. 13, 1141 (2001).
N. C. Coronel, M. J. Silva, S. O. Ferreira, et al., Chem. Sel. 4, 302 (2019).
A. V. Ivanov, T. V. Vasina, O. V. Masloboishchikova, et al., Russ. Chem. Bull. 49, 1726 (2000).
P. A. Korovchenko, N. A. Vladimirov, R. A. Gazarov, and L. M. Kustov, Russ. Chem. Bull. 52, 2376 (2003).
P. A. Korovchenko, R. A. Gazarov, A. Yu. Stakheev, and L. M. Kustov, Russ. Chem. Bull. 48, 1261 (1999).
L. M. Kustov, Top. Catal. 4, 131 (1997).
A. A. Davydov, IR Spectroscopy in the Chemistry of Oxide Surfaces (Nauka, Novosibirsk, 1984) [in Russian].
K. I. Hadjiivanov and G. N. Vayssilov, Adv. Catal. 47, 307 (2002).
F. Aubke and C. Wang, Coord. Chem. Rev. 137, 483 (1994).
N. Sheppard and T. T. Nguyen, Advances in Infrared and Raman Specroscopy, Ed. by R. E. Hester and R. J. H. Clark (Heyden and Son, London, 1978), Vol. 5, Chap. 2.
H. Bischoff, N. I. Jaeger, G. Schulz Ekloff, and L. Kubelkova, J. Mol. Catal. 80, 95 (1993).
C. Besoukhanova, J. Guidot, D. Barthomeuf, et al., J. Chem. Soc., Faraday Trans. 77, 1595 (1981).
A. A. Solomennikov and A. A. Davydov, Kinet. Katal. 25, 403 (1984).
J. A. Anderson, M. G. V. Mordente, and C. H. Rochester, J. Chem. Soc., Faraday Trans. I 85, 2983 (1989).
H. Heyne and F. C. Tomkins, Trans. Faraday Soc. 63, 1274 (1967).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Tkachenko, O.P., Gazarov, R.A. & Kustov, L.M. Studying the Stability of Supported Heteropoly Compounds Using Data from Diffuse Reflectance Infrared Spectroscopy. Russ. J. Phys. Chem. 95, 1560–1568 (2021). https://doi.org/10.1134/S0036024421080264
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421080264