Abstract
Solubilities, densities and refractive indices for the quaternary system KCl + KBO2 + K2SO4 + H2O at 298.15 K were determined using the isothermal dissolution equilibrium method. According to the experimental data, the diagrams of solubilities, densities, and refractive indices were plotted, respectively. The phase diagram consist of one invariant point, three isothermal dissolution curves, and three crystallization regions corresponding to potassium chloride (KCl), potassium metaborate hydrate (KBO2⋅1.33H2O), and potassium sulfate (K2SO4), respectively. The size of crystallization areas of salt is in the order K2SO4 > KCl > KBO2⋅1.33H2O, which indicates K2SO4 could be more easily separated from the system. The diagrams of solution density and refractive index changed regularly with changing concentration of KCl at 298.15 K.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Potassium and boron are important chemical raw materials, as they are widely used in fertilizer, pigment, pharmaceutical, paper, detergent, glass, and ceramics industries [1]. As the available amounts of solid ores are decreasing, the development of liquid potassium and boron mineral resources has become an inevitable tendency in future. Liquid potassium and boron mineral resources are mainly distributed in salt lake brine, seawater, geothermal water, and oil-field water. There are abundant salt lake brine resources in the Qaidam Basin, China with high contents of lithium, potassium, boron, the valuable brine mostly belong to the MgSO4 subtype brine as a seven-component system (Li+ + Na+ + K+ + Mg2+ + Cl- + \({\text{SO}}_{4}^{{2 - }}\) + borate + H2O), which is of great exploitation potential and strategic importance [2, 3].
It is well-known that phase diagrams and phase equilibria play an important role in guiding the comprehensive utilization of the valuable brine. To take full advantage of the brine resources, the phase equilibria of some subsystems containing potassium borate have been researched, such as (KBO2 + H2O) [4], (KBO2 + KOH + H2O) [4, 5], (LiBO2 + NaBO2/KBO2 + H2O) [6], (KCl/K2SO4 + KB5O8 + H2O) [7], (LiB5O8/NaB5O8 + KB5O8 + H2O) [8], (K2O + CaO + B2O3 + H2O) [9]. Although the phase equilibrium and phase diagrams of potassium borate-containing systems over a wide temperature range have been previously reported, research has mainly focused on ternary systems, the solubility data of more complex systems is seriously insufficient. The quarternary system (KCl + KBO2 + K2SO4 + H2O) is actually part of (K2O + B2O3 + K2SO4 + KCl + H2O) with fixed K2O/B2O3 ratio, which has significant value for separating and purifying potassium and boron salts from the brines. In this paper, the solubilities, densities, and refractive indices of the quaternary systerm (KCl + KBO2 + K2SO4 + H2O) at 298.15 K are presented.
EXPERIMENTAL
Apparatus and Reagents
A thermostat with magnetic stirring (XHC-500-6, Beijing Fortunejoy Sci. Technol. Co. Ltd.) was used to maintain the temperature with a precision of 0.01 K in the equilibrium experiments. The refractive index (nD) was measured using an Abbe refractometer (Abbemat 550, Anton Paar Co. Ltd., Austria) with an uncertainty within ±0.0001. Density (ρ) was determined using Anton Paar Digital vibrating-tube densimeter (DMA 4500, Anton Paar Co. Ltd., Austria) with an uncertainty of less than ±0.15 mg cm−3.
The reagents used in this study were of analytical grade and obtained from the Sinopharm Chemical Reagent Co. Ltd.: KCl (mass fraction >99.0) and K2SO4 (mass fraction >99.0). The potassium metaborate were synthesized in the lab [10], and the purity is 99.0 in mass fraction. Doubly deionized water (DDW, k < 1 × 10–4 S m–1, pH 6.60) were employed to prepare the series of samples and for chemical analysis.
Method
The isothermal dissolution equilibrium method was adopted in this work [11, 12]. On the basis of the phase equilibrium composition, salts and water of appropriate quantity were mixed together and loaded into a series of equilibrium bottles, and then placed in the magnetic stirring thermostatic bath and the temperature was set at set at 298.15 ± 0.1 K with 150 rpm to accelerate the achievement of equilibrium. At intervals, a 5.0 mL sample of the clarified solution was taken out for chemical analysis. When the difference between the concentrations of the two samples taken from the same sample was within ±0.3% in mass fraction, it indicated that the equilibrium had been achieved. Generally, it took approximately 14 days to reach the equilibrium state for the two systems. Then, liquid supernatant was taken out for quantitative chemical analysis, and the solid phases were also sampled and identified by X-ray diffractometry.
The \({\text{BO}}_{2}^{ - }\) ion concentration was measured in triplicate by alkali titration method with sodium hydroxide standard solution in the presence of mannitol and double indicator of methyl red and phenolphthalein and the relative standard uncertainty of the analytical results is no more than ±0.003. The Cl‒ ion was determined by volumetric titration with AgNO3 standard solution in the presence of potassium chromate as indicator with relative standard uncertainty of no more than ±0.003. The K+ ion concentration was determined in triplicate by the gravimetric method with sodium tetraphenylborate. The \({\text{SO}}_{4}^{{2 - }}\) ion was determined via gravimetric method with barium chloride; the relative standard uncertainty for the analytical results in triplicate was within ±0.005 in mass fraction [13].
RESULTS AND DISCUSSION
Phase Equilibrium of Quaternary System at 298.15 K
The experimental data on the solubilities, densities, and refractive indices of the quaternary system (KCl + KBO2 + K2SO4 + H2O) at 298.15 K are presented in Table 1. There are three boundary subternary systems in this quaternary system, which are (KCl + KBO2 + H2O), (KBO2 + K2SO4 + H2O), and (KCl + K2SO4 + H2O). A series of studies have been carried out on the stable phase equilibrium of these three ternary subsystems at different temperatures and relevant experimental data and phase diagrams have been obtained [7, 14, 15]. Solubilities of the invariant points for the three subternary systems are shown in Table 2. Among them, the ternary systems (KCl + KBO2 + H2O) and (KBO2 + K2SO4 + H2O) have not solubility data at 298.15 K in the literature; therefore, the solubilities of ternary system containing K2B4O7 and KB5O8 were listed for comparison in Table 2. The solid phases of potassium borate were showed K2B4O7⋅4H2O and KB5O8⋅4H2O, and the solubility of K2B4O7 and KB5O8 is much less than KBO2 [7, 14]. For the (KCl + K2SO4 + H2O) system, the solid phases and solubilities in the invariant point obtained in this work are in accordance with the literature [15].
In Table 1, w is the mass fraction, J(b) is the Jänecke index values of the component b, with J(KCl) + J(K2SO4) + J(KBO2) = 100. The calculating formula for J(b) is
The calculating formula for water concentration is
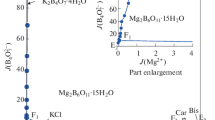
According to the experimental data of the Jänecke index J(b), the dry-salt phase diagram of this system at 298.15 K were plotted with solid line in Fig. 1. In Fig. 1, the stable phase diagram of the quaternary system at 298.15 K consists of one invariant points, three isothermal dissolution equilibrium curves and three crystalline fields corresponding to potassium chloride (KCl), potassium metaborate hydrate (KBO2⋅1.33H2O), potassium sulfate (K2SO4). The crystallization field decreases in order of K2SO4 > KCl > KBO2⋅1.33H2O, and the solubility of the corresponding salt increases sequentially. The five isothermal dissolution equilibrium curves correspond to AE (KBO2⋅1.33H2O + KCl), BE (K2SO4 + KCl), and CE (KBO2⋅1.33H2O + K2SO4), respectively. The invariant point E is composed of liquid + KCl + K2SO4 + KBO2⋅1.33H2O, with w(KCl) = 5.00, w(K2SO4) = 0.11, w(KBO2) = 39.03.
In order to fully reflect the existence of a certain point in the system, the diagram of the water-content vs composition was plotted in Fig. 2 according to its dry salt composition. It can be seen that the water content of quaternary system (KCl + KBO2 + K2SO4 + H2O) changes regularly with the change of KCl concentration at 298.15 K, and the water content maximizes at point E. Regarding isothermal dissolution equilibrium curve CE, the value of J(H2O) decreases from 140.27 to 126.55 with the increase of J(KCl), and increases gradually on the curve EB.
The solid phase was identified by X-ray diffraction (XRD) as shown in Fig. 3. The obtained X-ray diffraction pattern matches well with the standard diffraction pattern. It shows that salts KBO2⋅1.33H2O, K2SO4, and KCl coexist in this quaternary systerm (KCl + KBO2 + K2SO4 + H2O) at 298.15 K.
The solid phase of potassium borate in (KCl + K2B4O7 + H2O) and (K2SO4 + K2B4O7 + H2O) systems was showed K2B4O7⋅4H2O, and the solubility of K2B4O7 is much less than KBO2.
The phase equilibria of the quaternary system (KCl + K2B4O7 + K2SO4 + H2O) at 298.15 K has been reported [14]. For comparison purposes, it is shown in Fig. 4 along with data of (KCl + K2SO4 + KBO2 + H2O) system measured at 298.15 K. The diagrams show that there are remarkable differences in the reported phase field sizes and solid phase types. (1) There are three crystallization phase region present in (KCl + K2B4O7 + K2SO4 + H2O) system: KCl, K2SO4⋅H2O, and K2B4O7⋅4H2O; (2) potassium borate of the formula K2B4O7⋅4H2O is formed in (KCl + K2SO4 + K2B4O7 + H2O) system, while KBO2⋅1.33H2O generated in (KCl + K2SO4 + KBO2 + H2O) system, and the solubility of K2B4O7 is much less than KBO2; (3) the area of crystallization region of KCl and K2SO4⋅H2O decreased, while the K2B4O7⋅4H2O increased obviously in (KCl + K2SO4 + K2B4O7 + H2O) system.
A comparison of phase diagram for the quaternary system: (1) (KCl + KBO2 + K2SO4 + H2O) with (2) (KCl + K2SO4 + K2B4O7 + H2O) at 298.15 K [14].
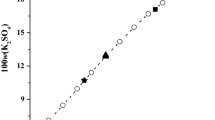
Based on the experimental data in Table 1, the diagrams for solution densities and refractive indices versus composition in the quaternary system (KCl + KBO2 + K2SO4 + H2O) at 298.15 K were drawn and are shown in Fig. 5. It was found that the solution densities and refractive indices changed regularly with the increasing of KCl concentration, which first increased slightly with the increasing concentration of KCl in the solubility isotherm curves of AE and CE, and reached the maximum value of 1.55085 g cm–3 and 1.4264 at the invariant point E, and then decreased sharply with the increasing concentration of KCl in the solubility isotherm curve of EB.
CONCLUSIONS
Solid–liquid phase equilibria for the quaternary system of (KCl + KBO2 + K2SO4 + H2O) at 298.15 K were studied using the isothermal dissolution equilibrium method, and the solubilities, densities, refractive indices were obtained for the first time. There are one invariant point, three univariant solubility curves and three crystallization regions corresponding to KCl, KBO2⋅1.33H2O, K2SO4, respectively. The size of crystallization areas of salt is in the order K2SO4 > KCl > KBO2⋅1.33H2O, which indicates K2SO4 could be more easily separated from the system. There are remarkable differences in the reported phase field sizes and solid phase types in comparison with the (KCl + K2B4O7 + K2SO4 + H2O) system, and the solubility of K2B4O7 is much less than KBO2. This phase diagram can be used to guide the separating process of KBO2, KCl, K2SO4 from the high concentration potassium-containing salt lake brine.
REFERENCES
D. L. Shen, X. P. Yu, Y. F. Guo, et al., Appl. Mech. Mater. 71, 2594 (2011).
X. Y. Zheng, M. G. Zhang, C. Xu, and B. X. Li, Salt Lakes of China (Chinese Science Press, Beijing, 2002).
X. Y. Zhao, L. Y. An, and X. Q. Tan, Ind. Miner. Process 5, 16 (2012).
O. Krol, J. Andrieux, J. J. Counioux, et al., in Proceedings of the JEEP 35th Conference on Phase Equilibria, 2009, p. 00023.
A. V. Churikov, K. V. Zapsis, V. V. Khramkov, et al., J. Chem. Eng. Data 56, 383 (2011).
S. Q. Wang, J. Yang, C. C. Shi, et al., J. Chem. Eng. Data 64, 3122 (2019).
Y. Yu, K. Y. Zhao, Y. F. Guo, et al., J. Solution Chem. 48, 1135 (2019).
K. R. Sun, K. Y. Zhao, L. Li, et al., J. Solution Chem. 48, 1105 (2019).
Z. Apagyi and L. J. Csetenyi, Cem. Concr. Res. 31, 1087 (2001).
L. X. Zhu, S. Y. Gao, B. Wang, et al., Chin. J. Inorg. Chem. 19, 333 (2013).
S. Q. Wang, Y. F. Guo, D. C. Li, et al., J. Chem. Eng. Data 60, 821 (2015).
S. Q. Wang, Y. F. Guo, D. C. Li, et al., Thermochim. Acta 601, 75 (2015).
Qinghai Institute of Salt Lakes of CAS, Analytical Methods of Brines and Salts, 2nd ed. (Chinese Science Press, Beijing, 1988).
Z. L. Zhang, S. H. Sang, M. Li, et al., Chin. Chem. Eng. 37, 46 (2009).
H. L. Silcock, Solubilities of Inorganic and Organic Compounds: Ternary and Multicomponent Systems of Inorganic Substances (Pergamon, New York, 1979).
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (22078247, U1707602, 21773170, and 21106103), the Natural Science Foundation of Tianjin (17JCYBJC19500), and the Yangtze Scholars and Innovative Research Team in University of Ministry of Education of China (IRT-17R81).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng Gu, Wang, Sq., Guo, Yf. et al. Solid–Liquid Phase Equilibrium in the Quaternary System KCl + KBO2 + K2SO4 + H2O at 298.15 K. Russ. J. Phys. Chem. 95, 717–723 (2021). https://doi.org/10.1134/S0036024421040087
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024421040087