Abstract
The heats of dissolution of crystalline picolinic acid in water and in potassium hydroxide solutions at 298.15 K are measured via direct calorimetry. The standard enthalpies of formation of the acid and its dissociation products in an aqueous solution are calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Pyridine monocarboxylic acids isonicotinic (а), nicotinic (b), and picolinic acids (с)

which have very interesting pharmacological properties, form an important group of anthelmintic drugs and vitamins [1]. These compounds participate in a number of important biochemical processes in a human organism [2]. For example, nicotinic acid is converted to nicotinamide adenine nucleotide, which serves as an intermediate in the processes of two-electron transfer in biochemical processes [3]. Pyridinecarboxylic acids can interact with ions of a number of metals [2]. The biological importance of picolinic, nicotinic, and isonicotinic acids, and particularly their complexes, was described in [4–9]. The biochemical importance of protolytic equilibria is obvious, especially when studying the transport functions of nicotinic acid. Vitamin PP penetrates the lipoprotein membrane due mainly to a diffusion mechanism when there is a concentration gradient of the molecular form of nicotinic acid, which turns into nicotinate ions at the pH of the intracellular medium [10].
Reliable data on acid ionization constants can be found in the literature [11–22]. These works were performed at different values of the ionic strength of the solution and with different background electrolytes. To be able to compare the values of the stepwise acid dissociation constants obtained by different authors, we recalculated the values of pK1 and pK2 to zero ionic strength.
The acid dissociation constants at zero ionic strength were calculated using the Davis equation [23] (for I < 0.5)
and by the equation (for I > 0.5):
where pKc and pK0 are negative logarithms of the concentration and thermodynamic dissociation constants; \({{\Delta }{{Z}^{2}}}\) is the difference between the squares of the charges of the reaction products and the initial materials; A is the constant of the Debye limit law, which is equal to 0.5107 at 25°C; δ is an empirical coefficient; and I is the ionic strength of the solution. The thermodynamic stepwise dissociation constants were also determined graphically [24]:
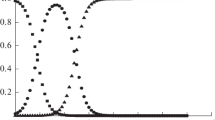
After processing the literature data (stepwise dissociation constants), the most probable values of the thermodynamic dissociation constants can be taken as 298.15 K: pK\(_{1}^{0}\) = 0.85 ± 0.03, pK\(_{2}^{0}\) = 5.18 ± 0.05. Figure 1 shows the equilibrium diagrams in an aqueous solution of picolinic acid, constructed on the basis of calculations of the equilibrium composition of acid solutions at different pH values using the KEV program [25].
The aim of this work was to determine standard enthalpies of formation for picolinic acid and its dissociation products in an aqueous solution according to the thermal effects of acid dissolution in water and in aqueous KOH solutions at 298.15 K.
EXPERIMENTAL
Picolinic acid purchased from Sigma-Aldrich (content of the main substance, 99.0%) was used in the work without further purification. Acid solutions were prepared by dissolving weighed amounts of the reagent in freshly prepared doubly distilled water immediately before each experiment. Carbonate-free KOH and HNO3 solutions were prepared from reagents of chemically pure grade according to the standard procedure in [26]. The operation of the calorimetric unit [27] was checked according to a generally accepted calorimetric standard, the heat of dissolution of crystalline potassium chloride in water. KCl was purified via double recrystallization of the chemically pure reagent from doubly distilled water, and dried to constant weight in an oven at 393.15 K. The agreement between the experimentally obtained heats of dissolution of KCl(cr.) in water ΔsolH(∞H2O) = 17.25 ± 0.06 kJ/mol and the most reliable literature data [28] shows there was no notable systematic error in the operation of the calorimetric setup.
RESULTS AND DISCUSSION
Protonation of the anionic acid form (L−) proceeds in two stages:
where HL± is the zwitterionic form; H2L+ is the cationic form (protonated amino group); and L− is the anionic form (ionized carboxyl group).
The standard enthalpies of formation of picolinic acid solution at different dilutions were calculated with the equation
where ΔfH°(H2L, cr., 298.15 K) = −343.8 ± 1.8 kJ/mol is the standard enthalpy of crystalline picolinic acid formation [29, 30]; ∆solH(HL±, 298.15 K) is the heat of dissolution of the acid at different dilutions (Table 1).
The enthalpies of acid formation in an aqueous solution in the considered range of concentrations are virtually independent of the dilution, which is not surprising for such strong dilutions.
The standard enthalpy of formation of a hypothetically non-dissociated acid molecule at final dilutions in an aqueous solution was found with the equation
where α1 and α2 are the fractions of particles H2L+ and L−, respectively; \({{\Delta }_{{{\text{dis}}}}}H_{1}^{ \circ }\) and \({{\Delta }_{{{\text{dis}}}}}H_{2}^{ \circ }\) are the enthalpies of stepwise acid dissociation, determined earlier in our laboratory: \({{\Delta }_{{{\text{dis}}}}}H_{1}^{ \circ }\) = 13.61 ± 0.36 kJ/mol and \({{\Delta }_{{{\text{dis}}}}}H_{2}^{ \circ }\) = 2.02 ± 0.25 kJ/mol. The equilibrium composition of the solutions was calculated using the KEV program [25]. It showed that the fractions of particles HL± and L− were no more than 3 × 10−3 and 5 × 10−7, respectively. The total contribution from the second and third terms on the right-hand side of Eq. (7) thus does not exceed 0.03 kJ/mol and remains virtually unchanged in the considered range of concentrations, so these contributions can be ignored.
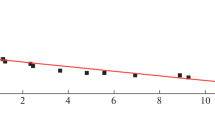
The standard enthalpy of formation of acid in a hypothetical undissociated state with infinite dilution was found by extrapolating the values obtained according to Eq. (7) to zero molality m of the solution. Points in coordinates −∆fH°(H2L, sol., nH2O, hyp. nondis.; 298.15 K)−m satisfactorily fit on a straight line.
The following values were obtained using the least squares method:
Standard enthalpies of particle formation (H2L+, L–) in an aqueous solution were determined using data on the standard enthalpies of acid formation in a hypothetical undissociated state and values of enthalpies of acid stepwise dissociation.
We used the below procedure for independent determination of the standard enthalpy of formation of particles L− and other products of the dissociation of picolinic acid in an aqueous solution. A series of experiments was performed to determine the enthalpies of acid dissolution in alkali solutions with an equivalent ratio of at least 1 : 2.
The process of dissolution in a KOH solution can be presented as
The reliability of such a dissolution scheme is confirmed by the diagram of equilibrium in an aqueous solution of picolinic acid. Figure 1 shows that particles L− predominate when pH >6.5. The pH value was much higher under the conditions of our experiment, since a double excess of alkali was taken. Calculations showed that the completeness of reaction (8) was at least 99.9% for all calorimetric experiments. The experimental data are presented in Table 2.
Since difference ΔZ 2 = 0 in reaction (8) between the squared charges of the reaction products and the initial components is zero, the thermal effects of acid dissolution at zero ionic strength were calculated according to the equation [24]
where ΔrH(8) and \({{\Delta }_{{\text{r}}}}H_{{(8)}}^{\circ }\) are the thermal effects of process (8) at finite and zero values of ionic strength; i is an empirical coefficient; and I is the ionic strength of the solution.
Using obtained values \({{\Delta }_{{\text{r}}}}H_{{(8)}}^{\circ }\) and values ∆fH°(OH‒, sol., H2O, 298.15 K), ∆fH°(H2O, g, 298.15 K), recommended in [31], we calculated the standard enthalpy of formation of a deprotonated acid anion:
Standard enthalpy of particle formation HL± in the standard hypothetical undissociated state was also calculated with the equation
Standard enthalpy of particle formation H2L was found using the relation
The values of the standard enthalpies of formation of picolinic acid and the products of its dissociation in an aqueous solution are key values in the thermochemistry of these compounds, opening up the possibility of rigorous thermodynamic calculations in systems containing these compounds (Table 3).
REFERENCES
Z. Vargova, V. Zeleoak, I. Cisaova, and K. Gyoryova, Thermochim. Acta 423, 149 (2004).
J. L. York, in Textbook of Biochemistry with Clinical Correlations, Ed. by T. M. Delvin (Wiley, New York, 1992), p. 135.
K. Kiec-Kononowicz and E. Szymaska, Il Farmaco 57, 909 (2003).
W. Wang, A. Basinger, B. Shane, et al., Am. J. Physiol. Endocrinol. Metab. 280, 540 (2001).
I. Berg, B. V. L. Potter, G. W. Mayr, and A. H. Guse, J. Cell Biol. 150, 581 (2000).
M. Sulanc and I. Emre, J. Appl. Entomol. 124, 151 (2000).
B. Ahrén, Acta. Physiol. Scand. 171, 161 (2001).
P. Koczo, J. Cz. Dobrowolski, W. Lewandowski, and A. P. Mazurek, J. Mol. Struct. 655, 89 (2003).
T. Jakusch, K. Gajda-Schrantz, and T. Kiss, J. Inorg. Biochem. 100, 1521 (2006).
V. B. Spirichev, Fortification of Food with Vitamins and Minerals. Science and Technology (Sib. Univ., Novosibirsk, 2005) [in Russian].
M. Paula, M. Meier, and B. Szpoganicz, J. Coord. Chem. 46, 491 (1999).
J-C. Halle, J. Lelievre, and F. Nerrier, Can. J. Chem. 74, 613 (1996).
A. Asuero, M. Navas, A. Recamales, and M. Herrador, Microchim Acta, 395 (1986).
E. Chiacchieriuni, Ann. Chim. (Rome) 67, 547 (1977).
V. Lubes, J. Solution Chem. 34, 899 (2005).
E. Kiss, K. Petrohan, D. Sanna, and E. Garriba, Polyhedron 19, 55 (2000).
J. Loring, M. Karlsson, W. Fawcett, and W. Casey, Geochim. Cosmol. Acta 64, 4115 (2000).
R. Gelb and J. Alper, J. Chem. Eng. Data 43, 383 (1998).
M. Ignaczak and G. Andrijewski, Pol. J. Chem. 69, 716 (1995).
O. Jons and E. Johansen, Inorg. Chim. Acta 151, 129 (1988).
M. Seleim and K. Idriss, Analyst 112, 1685 (1987).
N. N. Kuranova, S. V. Dushina, and V. A. Sharnin, Russ. J. Phys. Chem. A 84, 792 (2010).
C. Davies, J. Chem. Soc., 2093 (1938).
V. P. Vasil’ev, Thermodynamic Properties of Electrolyte Solutions (Vysshaya Shkola, Moscow, 1982), pp. 200, 213 [in Russian].
A. N. Meshkov and G. A. Gamov, Talanta 198, 200 (2019).
P. P. Korostelev, Preparation of Solutions in Analytic Chemistry (Akad. Nauk SSSR, Moscow, 1962), p. 398 [in Russian].
A. I. Lytkin, V. V. Chernikov, O. N. Krutova, and D. K. Smirnova, Russ. J. Gen. Chem. 88, 954 (2018).
D. G. Archer, J. Phys. Chem. Ref. Data 28, 1 (1999).
A. I. Gubareva, I. A. Bukhtoyarova, P. A. Gerasimov, and S. N. Gadzhiev, Zh. Prikl. Khim. 63, 448 (1990).
M. D. M. C. Ribeiru da Silva, J. Chem. Thermodyn. 30, 869 (1998). https://doi.org/10.1006/jcht.1998.0353
Thermal Constants of Substances, Ed. by V. P. Glushko (VINITI, Moscow, 1965–1971), No. 3 [in Russian].
Funding
This work was performed as part of a State Task for the Research Institute of Thermodynamics and Kinetics of Chemical Processes, Ivanovo State University of Chemical Technology, project no. FZZW-2020-0009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lytkin, A.I., Chernikov, V.V., Krutova, O.N. et al. Standard Enthalpies of Formation of Picolinic Acid and the Products of Its Dissociation in an Aqueous Solution. Russ. J. Phys. Chem. 94, 2569–2573 (2020). https://doi.org/10.1134/S003602442012016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442012016X