Abstract
A series of Cu-SSZ-13 catalysts with different Si/Al molar ratio (10.6, 13.0, and 16.0) were prepared via one-pot method and investigated for the selective catalytic reduction by NH3. It was found that Cu-SSZ-13 with Si/Al ratio of 13.0 exhibited the best NH3-SCR performance with more than 94% NO conversion from 200 to 550°C. The influence of Si/Al molar ratio on the physical structure, redox behavior, surface acidity and Cu species distribution on the Cu-SSZ-13, together with the NH3 and nitrate adsorption species on the surface of Cu-SSZ-13 were characterized by N2-BET, XRD, NH3-TPD, H2-TPR, XPS, and in-situ DRIFTS, respectively. The characterization results indicated that the BET surface area, zeolite structure, surface acidity, redox properities, Cu2+ concentration, NH3 and nitrate adsorption species on the catalyst surface were closely related to the Si/Al ratio, all of these factors played important roles in the NH3-SCR performance for the Cu-SSZ-13.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Nitrogen oxides (NOx) in the air can cause serious environmental issues, such as acid rain, ozone depletion, haze and photochemical Smog et al. [1]. Hence, NOx shows great hazards to the environment and people’s health. The majority of NOx is generated from stationary (power plants) and mobile sources (diesel engines). In order to control NOx emissions, most countries have implemented strict emission standards. Selective catalytic reduction with NH3 (NH3-SCR) has been accepted as the most effective and widely applied technology for the abatement of NOx. As the core of the NH3-SCR technology, catalyst played a key role during the denitrification process. For the diesel exhaust, the traditional commercial V2O5–WO3 (MoO3)/TiO2 catalyst cannot meet the requirements due to its own defects, such as the narrow reaction temperature window, the toxicity of V2O5 and the inadequate hydrothermal stability [2, 3]. In order to reduce the NOx emissions from diesel exhaust, great efforts have been made to develop novel catalysts.
Up to now, various kinds of catalysts have been reported to be active for the abatement of NOx produced from the diesel engine. Among these catalysts, Cu-SSZ-13 has received more research interests compared to the other catalysts in recent years due to its excellent NH3-SCR catalytic activity and good hydrothermal stability. Kwak et al. observed that Cu ion-exchanged SSZ-13 zeolite showed higher catalytic activity and N2 selectivity compared with Cu-beta and Cu-ZSM-5 zeolites in the NH3-SCR [4]. However, the general preparation method of Cu-SSZ-13 catalyst requires expensive structure directing agent N,N,N-trimethyl-1-adamantammonium hydroxide, which limits its wide application in practice [5]. Ren et al. designed low-cost copper–amine complex as the template for one-pot synthesis of Cu-SSZ-13 zeolite [6]. Xie et al. further confirmed that Cu-SSZ-13 synthesized with one-pot method is a promising candidate as an NH3-SCR catalyst for the NOx abatement from diesel vehicles [7]. Corma et al. synthesized the Cu-SSZ-13 zeolite with one-pot methodology based on the cooperative use of Cu-tetraethylenepentamine complex and N,N,N-trimethyl-1-adamantamonium as organic structure-directing agents, which also demonstrates excellent catalytic activity and good hydrothermal stability for the NH3-SCR [8].
Meanwhile, many effects have been put to study the reaction mechanism, the distribution of active sites and how to further improve the NH3-SCR performance of Cu-SSZ-13 [9–13]. Nevertheless, the effect of Si/Al molar ratio on the catalytic activity of one-pot prepared Cu-SSZ-13 is much less reported compared with the other investigations. Gao et al. prepared SSZ-13 with different Si/Al ratios by the traditional method with N,N,N-trimethyl-1-adamantammonium hydroxide as structure directing agent and then supported Cu species with ion exchange method. They found that Cu ion location and Brønsted acidity is closely related to the Si/Al ratio [14]. Fan et al. synthesized Cu-SSZ-13 catalysts with three Si/Al ratios by the one-pot method according to Corma’s report with Cu-tetraethylenepentamine complex and N,N,N-trimethyl-1-adamantamonium as template, the results exhibited that the Si/Al ratio not only influence on the nature and the distribution of Cu species but also affect the acidity of the catalysts [15]. In the present study, three Cu-SSZ-13 catalysts with different Si/Al ratios were prepared based on Ren’s method with cheap Cu-tetraethylenepentamine complex as template. The NH3-SCR catalytic activity was evaluated to find the relationship with Si/Al ratio. Moreover, the XRD, N2-BET, MP-AES, NH3-TPD, H2-TPR, XPS, and in situ DRIFTS were also performed to characterize the physicochemical properties of the Cu-SSZ-13 catalysts.
EXPERIMENTAL
Catalyst Preparation
Cu-SSZ-13 zeolite with different Si/Al ratios were prepared via the one-pot hydrothermal method reported by Ren et al. with the molar ratio of 5.0Na2O : xAl2O3 : 10SiO2 : 2.0Cu-TEPA : 200H2O [6]. In a typical synthesis procedure of Cu-SSZ-13 catalyst with Si/Al ratio of 13.0, NaAlO2 and NaOH were added into deionized water. The solution was stirred for 30 min at room temperature. Then, CuSO4 · 5H2O and TEPA were added and continuously stirred for 30 min. Then, 30 wt % silica sol was added to the above solution and stirred for another 3 h. The resulting solution was transferred into 50 mL Teflon-lined autoclaves and heated at 140°C for 4 days. The product was filtered and washed with water and then heated at 110°C for 12 h. The as-prepared product was treated with 1 M NH4NO3 solution at 80°C for twice, followed by washing and drying. Then, the sample was calcined at 600°C for 6 h to obtain the final product of Cu-SSZ-13-13.0, where “13.0” denoted Si/Al molar ratio. For the preparation of other Cu-SSZ-13 catalysts with Si/Al ratios of 10.6 and 16.0, the amount of the NaAlO2 was only changed during the synthesis process.
Catalyst Characterization
Power XRD was performed on a Rigaku D/max-RC diffractometer with CuKα radiation at 40 kV and 25 mA (λ = 0.15418 nm). The intensities were recorded within the scanning range of 5°–50° at the speed of 6 deg/min.
The N2 sorption isotherm was measured on an ASAP2020 instrument. The samples were first degassed at 300°C for 6 h. The specific area was calculated according to the BET method and the micropore volume was determined by the t-plot method.
The Cu contents of the catalysts were determined using an Agilent 4200 MP-AES. Catalysts were digested for 16 h in 10 mL of aqua regia and then diluted to 100 mL with de-ionized water. Every sample was measured three times and the average result was reported.
H2-TPR and NH3-TPD were conducted on a Micromeritics Autochem-II instrument. In a typical H2-TPR experiment, 50 mg sample was placed in a quartz tube and pretreated in flowing He at 300°C for 1 h. The sample was then cooled to 50°C under flowing He. The sample was reduced starting at 50°C and increasing up to 900°C in a gas mixture of 10% H2/He at 10 K/min. In the case of NH3-TPD, the samples were pretreated at 400°C under He for 1 h, then cooled to 50°C and saturated with 10% NH3/He for 1 h, followed by purging with He for 30 min. Finally, the sample was heated from 50 to 800°C at a rate of 10 K/min.
X-ray photoelectron spectroscopy measurements were carried out on a PHI-1600ESCA System XPS spectrometer (Perkin-Elmer, USA) using a non-monochromatic MgKα radiation, operated at 15 kV and under 10–7 Pa pressure with photoelectron energy set at 1254 eV. The reported binding energies were referenced to the C 1s binding energy of 284.6 eV.
The in situ DRIFTS experiments were carried out on an FTIR spectrometer (VERTEX 70, Bruker) equipped with an in situ diffuse reflection chamber and an MCT detector cooled by liquid nitrogen. Prior to each test, the catalyst was finely ground and heated at 500°C for 60 min in a flow of 10% O2/N2 and then cooled down to 200°C to get a background spectrum. Then the background spectrum was subtracted from the sample spectrum. All the spectra were collected by accumulating 100 scans at a resolution of 4 cm–1.
Catalytic Performance Tests
The NH3-SCR activity measurements were carried out in a fixed bed quartz microreactor under atmospheric conditions. The catalysts were sieved with 40–60 mesh. The model flue gas composition: 500 ppm NO, 500 ppm NH3, 5% O2, and balance N2. The total flow rate was 200 mL min–1 yielding the GHSV of 100 000 h–1. The concentrations of NH3, NO, NO2, and N2O in the inlet and outlet gas were measured by an online FTIR spectrometer (MKS MultiGas 2030HS). The data were collected after 30 min when the SCR reaction had reached a steady state. Little amount of N2O was detected in our test conditions. The NO conversion (γ) was calculated as follows:
RESULTS AND DISCUSSION
Catalytic Performance
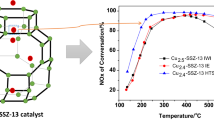
The catalytic activities of the Cu-SSZ-13 catalysts with different Si/Al molar ratio (10.6, 13.0, 16.0) were tested in the temperature range of 100–550°C with the GHSV of 100 000 h–1. As shown in Fig. 1, the Cu-SSZ-13-10.6 showed more than 95% NO conversion from 200 to 400°C. NO conversion decreased very quickly when the temperature above 400°C or below 200°C. With the increase in Si/Al molar ratio from 10.6 to 13.0, the high temperature NO conversion was kept above 94% even up to 500°C. Meanwhile, the low temperature NO conversion was also enhanced obviously. However, further increasing the Si/Al molar ratio to 16.0 resulted in an obvious decline of catalytic activity in the temperature range of 300–550°C. Therefore, it can be found that Cu-SSZ-13 catalyst with Si/Al molar ratio of 13.0 showed the best NH3‑SCR performance and Si/Al molar ratio was closely related to the catalytic activity.
Catalyst Physicochemical Characterization
Table 1 summarized the pore structure parameters and Cu concentrations of all the Cu-SSZ-13 catalysts. The BET surface area of Cu-SSZ-13-13.0 is higher than that of Cu-SSZ-13-10.6 and Cu-SSZ-13-16.0, yet the NH3-SCR performance of the former is much higher than that of the latter, indicating that the structural parameters and catalytic reactivity of the catalysts are influenced by the Si/Al molar ratio. The contents of Cu in the catalysts were also measured by MP-AES and the results were listed in Table 1. It could be found that the content of Cu in the catalyst did not change significantly with the change of Si/Al molar ratio.
The XRD patterns of Cu-SSZ-13 with different Si/Al molar ratios were shown in Fig. 2. The Cu-SSZ-13-10.6 and Cu-SSZ-13-13.0 displayed the typical XRD patterns of zeolites with the CHA framework topology [3]. As the Si/Al molar ratio was increased to 16.0, not only the peaks attributed to CHA structure were shifted to the higher position slightly but the intensities of principal diffraction peaks became weak and unclear, which is contrary to Fan’s report [15]. Meanwhile, it could be found that no diffraction peaks due to copper species were observed on these three Cu-SSZ-13 samples, indicating that coppers species should be in good dispersion or the particles of Cu species are too small to be detected.
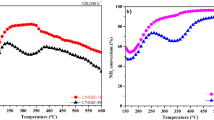
The reducibilities of Cu-SSZ-13 catalysts with different Si/Al molar ratio were measured by using H2-TPR and the results are shown in Fig. 3. It could be found that only a very wide peak ranging from 300 to 850°C was detected on the Cu-SSZ-13-10.6 catalyst. When the Si/Al molar ratio was increased to 13.0, three peaks were detected at 219, 340, and 877°C, respectively. Based on the previous studies, the peak at 219°C was attributed to the reduction of unstable Cu2+ inside the cage of CHA structure to Cu+, the peak at 340°C was ascribed to the reduction of stable Cu2+ located in six-membered rings to Cu+ and the peak at 876°C was due to the reduction of Cu+ to Cu0 [5]. For the Si/Al molar ratio is 16.0, the low temperature reduction peaks were shifted from 219 and 340°C to 415 and 580°C, respectively. However, there is no high temperature reduction peak appearing on the catalyst above 750°C, it could be found that with the Si/Al molar ratio was further increased the redox performance was inhibited obviously and the Cu-SSZ-13 catalyst with Si/Al molar ratio of 13.0 exhibited the optimal redox activity. The H2-TPR results suggested that the redox capacity of the Cu-SSZ-13 is closely related with the Si/Al molar ratio.
According to the previous reports, the acidity of the zeolite-based catalyst is strongly affected by the Si/Al molar ratio and then influences the NH3-SCR performance [16]. NH3-TPD was carried out to determine the surface acidity of the Cu-SSZ-13 catalysts with different Si/Al molar ratios. As shown in Fig. 4, four desorption peaks were detected on the Cu-SSZ-13-10.6, the peak at 122°C can be assigned to the weakly adsorbed NH3 on the weak Lewis acid sites. The peaks at 263 and 431°C could be due to NH3 adsorbed at strong Brønsted acid sites. The peak at 704°C could be attributed to strong acid sites originated from the highly disperse copper species on the catalyst. When the Si/Al molar ratio was increased to 13.0, the peak area of the total ammonia desorption was increased significantly. Especially in the low temperature region (<250°C), the corresponding peak area due to the weak Lewis acidity was increased evidently, which indicated that the improvement of NH3-SCR catalytic activity at low temperature is closely related to the enhancement of the surface acidity. However, when the Si/Al molar ratio was further increased to 16.0, the total ammonia desorption peak area decreased significantly, which might be related to the destruction of the chemical structure of Cu-SSZ-13. According to the NH3-TPD results, it can be seen that the concentrations of surface acid sites on the Cu-SSZ-13 was closely related to the Si/Al molar ratio, which also played an important role during the NH3-SCR performance.
XPS spectra was conducted to explore the chemical environment of Cu species on the surface of Cu-SSZ-13 with different Si/Al molar ratios. Cu 2p spectra of the Cu-SSZ-13 catalysts were depicted in Fig. 5. All samples displayed the typical structure for Cu species with Cu 2p3/2, Cu 2p1/2 and shake-up signals, respectively. The Cu 2p3/2 contained two peaks at 936.2 and 933.1 eV, which could be attributed to CuO and Cu2+. The shake-up peak at the binding energy of 944 eV could also be assigned to Cu2+ [17]. It could be found that the peaks due to Cu2+ were enhanced with the increase of Si/Al molar ratio, which also indicated that the amount of Cu2+ was increased. These results also confirmed that the distribution of copper species on the surface of Cu-SSZ-13 was influenced by Si/Al molar ratio.
For the purposes of examining the NH3 and nitrate adsorbed species on the Cu-SSZ-13 catalysts after changing the Si/Al molar ratio, the in situ DRIFTS of NH3 and NO + O2 adsorption at 200°C were performed, respectively. As it is shown in Fig. 6a, after NH3 adsorption, some peaks appeared on the surface of Cu-SSZ-13-10.6. The bands at 1268, 1309, and 1622 cm–1 are attributed to coordinated NH3 on Lewis acid sites [18–20], while the band at 1452 cm–1 could be assigned to ionic NH4+ species on Brønsted acid site [21]. The bands at 3178, 3265, and 3337 cm–1 are assigned to N–H stretching vibration modes [22]. When the Si/Al molar ratio was increased from 10.6 to 13.0, some new peaks at 1126, 1232, 1412, 1635 cm–1 were detected on its surface after NH3 adsorption. Based on the previous reports, the bands at 1126, 1309, and 1635 cm–1 are ascribed to the NH3 vibrations on Lewis acid sites [20, 23, 24], the band at 1232 cm–1 is due to the coordinated NH3 [25], the band at 1412 cm–1 is assigned to the NH\(_{4}^{ + }\) on the Brønsted acid site [26]. Meanwhile, the intensities of the peaks increased, which indicated that the surface acidity of the catalyst was enhanced. However, when the Si/Al molar ratio was further increased to 16.0, only NH\(_{4}^{ + }\) (1454 and 1665 cm–1) bands were observed on the Cu-SSZ-13-16.0 [21, 27], besides the intensity of the peak showed a significant decrease. Hence, it can be found that in situ DRIFTS NH3 adsorption results were consistent with the NH3-TPD results. When the NO and O2 were introduced into the DRIFT cell, almost no band was detected on the Cu-SSZ-13-10.6. When the Cu-SSZ-13-13.0 catalyst was exposed to NO + O2, three distinct bands appeared at 1617, 1594, and 1573 cm–1, which are respectively assigned to NO2 ad-species and bridged nitrate [28–30]. Over the Cu-SSZ-13-16.0, several new bands at 1275, 1362, 1375, and 1450 cm–1 were detected, which can be attributed to the monodentate nitrate and nitrate species adsorbed on transition metal [3, 31, 32]. According to the above analysis results, the number and the intensities of the adsorbed species on the Cu-SSZ-13 were closely related to the Si/Al molar ratio.
In conclusion, the effects of Si/Al molar ratio on the NH3-SCR performance of Cu-SSZ-13 was investigated in this work. The result showed that the Cu-SSZ-13 with Si/Al ratio of 13.0 exhibited the best catalytic activity. According to the characterization results, it could be found that the BET surface area, the redox capacity and the surface acidity reach the maximum value when the Si/Al molar ratio equals to 13. Meanwhile, the Cu species distribution, reactant NH3 adsorption states and the nitrates species on the catalyst surface are also affected by the Si/Al molar ratio. All of these results demonstrated that Si/Al molar ratio of Cu-SSZ-13 zeolite catalyst played an important role in the NH3-SCR performance.
REFERENCES
H. Bosch and F. Janssen, Catal. Today 2, 369 (1988).
X. Li and Y. Li, J. Mol. Catal. A: Chem. 386, 69 (2014).
T. Zhang, J. Li, J. Liu, D. Wang, Z. Zhao, K. Cheng, and J. Li, AIChE J. 61, 3825 (2015).
J. H. Kwak, R. G. Tonkyn, D. H. Kim, J. Szanyi, and C. H. Peden, J. Catal. 275, 187 (2010).
R. Zhang, Y. Li, and T. Zhen, RSC Adv. 4, 52130 (2014).
L. Ren, L. Zhu, C. Yang, Y. Chen, Q. Sun, H. Zhang, C. Li, F. Nawaz, X. Meng, and F. Xiao, Chem. Commun. 47, 9789 (2011).
L. Xie, F. Liu, L. Ren, X. Shi, F. Xiao, and H. He, Environ. Sci. Technol. 48, 566 (2013).
R. Martínez-Franco, M. Moliner, J. R. Thogersen, and A. Corma, ChemCatChem 5, 3316 (2013).
S. A. Bates, A. A. Verma, C. Paolucci, A. A. Parekh, T. Anggara, A. Yezerets, W. Schneider, J. Miller, W. Delgass, and F. H. Ribeiro, J. Catal. 312, 87 (2014).
L. Xie, F. Liu, X. Shi, F. Xiao, and H. He, Appl. Catal. B: Environ. 179, 206 (2015).
T. Zhang, F. Qiu, H. Chang, X. Li, and J. Li, Catal. Sci. Technol. 6, 6294 (2016).
U. Deka, A. Juhin, E. A. Eilertsen, H. Emerich, M. A. Green, S. T. Korhonen, and A. M. Beale, J. Phys. Chem. C 116, 4809 (2012).
X. Liu, Y. Li, and R. Zhang, RSC Adv. 5, 85453 (2015).
F. Gao, N. M. Washton, Y. Wang, M. Kollár, J. Szanyi, and C. H. Peden, J. Catal. 331, 25 (2015).
C. Fan, Z. Chen, L. Pang, S. Ming, X. Zhang, K. B. Albert, P. Liu, H. Chen, and T. Li, Appl. Catal. A 550, 256 (2018).
J. Wang, T. Yu, X. Wang, G. Qi, J. Xue, M. Shen, and W. Li, Appl. Catal. B: Environ. 127, 137 (2012).
J. Wang, Z. Peng, H. Qiao, H. Yu, Y. Hu, L. Chang, and W. Bao, Ind. Eng. Chem. Res. 55, 1174 (2016).
Y. Zhang, X. Zhu, K. Shen, H. Xu, K. Sun, and C. Zhou, J. Colloid Interface Sci. 376, 233 (2012).
C. H. Lin and H. Bai, Appl. Catal. B: Environ. 42, 279 (2003).
C. Qiu, D. Pang, C. Zhang, J. Meng, R. Zhu, and F. Ouyang, Appl. Surf. Sci. 357, 189 (2015).
W. Li, M. Sirilumpen, and R. T. Yang, Appl. Catal. B: Environ. 11, 347 (1997).
G. Busca, M. A. Larrubia, L. Arrighi, and G. Ramis, Catal. Today 107, 139 (2005).
K. Shen, Y. Zhang, X. Wang, H. Xu, K. Sun, and C. Zhou, J. Energy Chem. 22, 617 (2013).
B. Thirupathi and P. G. Smirniotis, Appl. Catal. B: Environ. 110, 195 (2011).
Z. Liu, Y. Liu, Y. Li, H. Su, and L. Ma, Chem. Eng. J. 283, 1044 (2016).
F. Cao, S. Su, J. Xiang, P. Wang, S. Hu, L. Sun, and A. Zhang, Fuel 139, 232 (2015).
Y. Yu, J. Chen, J. Wang, and Y. Chen, Chin. J. Catal. 37, 281 (2016).
Q. Li, H. Gu, P. Li, Y. Zhou, Y. Liu, Z. Qi, Y. Xin, and Z. Zhang, Chin. J. Catal. 35, 1289 (2014).
Z. Liu, H. Su, B. Chen, J. Li, and S. Woo, Chem. Eng. J. 299, 255 (2016).
Z. Zhang, L. Chen, Z. Li, P. Li, F. Yuan, X. Niu, and Y. Zhu, Catal. Sci. Techol. 6, 7151 (2016).
X. Wang, X. Li, Q. Zhao, W. Sun, M. Tade, and S. Liu, Chem. Eng. J. 288, 216 (2016).
S. Yang, C. Wang, J. Li, N. Yan, L. Ma, and H. Chang, Appl. Catal. B Environ. 110, 71 (2011).
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (no. 21606162).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiangjiang Feng, Shi, D., Xu, Z. et al. Selective Catalytic Reduction of NO by NH3 over One-Pot Prepared Cu-SSZ-13 Catalysts with Different Si/Al Molar Ratio. Russ. J. Phys. Chem. 94, 1797–1803 (2020). https://doi.org/10.1134/S003602442009006X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602442009006X