Abstract
Bi/Bi2O2SiO3/Bi2WO6 composite photocatalysts were synthesized via a simple reduction method with using ethylene glycol (EG) as reducing agent. Compared to the pure semiconductor, Bi/Bi2O2SiO3/Bi2WO6 showed the highest photoactivity in degradation of rhodamine B (RhB) and ciprofloxacin (CIP) under visible light. The enhanced phtocatalytic performance can be primarily attributed to the improved visible light absorption and the effective separation of the charge carries by introducing Bi2O2SiO3 and Bi produced surface plasmon resonance (SPR) effect, which is confirmed by UV–Vis diffuse refectance spectroscopy (DRS) and the photoelectrochemical measurements. In addition, cycle experiments explored the reliable stability and reusability for catalyst. Moreover, radical species trapping experiments demonstrated that holes (h+) played crucial role in photodegradation process. This study may help promote the development of eliminate organic pollutants and environmental protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Recently, photocatalysts based on semiconductors received great attention as promising materials for environmental purification and solar energy utilization [1–3]. In various semiconductor photocatalysis, much attention has been given to Bi-based photocatalysts [4, 5]. As a visible-light response photocatalyst, Bi2WO6 possesses many advantages such as unique layered structure, stable properties and so on [6, 7], but it still need further improvements due to the low charge separation efficiency [8, 9].
To date, a lot of Bi-based heterojunction with Bi2WO6 enhanced visible light-driven photocatalysts were reported, such as Bi2S3/Bi2WO6 [10], Bi2WO6/BiOBr [11], Bi2Fe4O9/Bi2WO6 [12], and BiVO4/Bi2WO6 [13]. Yang et al. [14] fabricated a composite of Bi2WO6 and BiOCl with a high activity in photodegradation of RhB, which is attributed to Bi2WO6/BiOCl heterojunctions suppressing the recombination of the photoinduced electron–hole pairs. Therefore, it provides an idea that designing a composite photocatalyst by connecting Bi2WO6 with other semiconductors could be used to enhance the photocatalytic performance, but the molten salt method will cause larger expenses. Bi2O2SiO3, as a highly active ultraviolent photocatalyst, has attracted more attention recently [15–17]. However, Bi2O2SiO3 can only be applied in the ultraviolet region because of the wide bandgap (~3.5 eV) [18, 19], it is necessary to take effective measures to expand the photo-response range of Bi2O2SiO3. Lu et al. [20] prepared Bi2O3/Bi2O2SiO3 heterojunction with enhanced photocatalytic performance. The reported system was prepared by a facile one-step calcination method. However, Bi2O3 at high temperatures is prone to crystal phase transition. Therefore, the narrow energy band of Bi2O3 is similar to Bi2WO6, and wide energy band of BiOCl is similar to Bi2O2SiO3, we can consider fabricating Bi2O2SiO3/Bi2WO6 composites due to the matchable energy levels and lattices.
In addition, the incorporation of noble metals (Ag, Au, Pt, et al.) onto Bi2WO6 could also expanded the light response range and promote the effective separation of photogenerated charge owing to the SPR effect [21, 22]. Unfortunately, these metals are expensive and not easy to collect. Recently, scientists found that metallic Bi also show the SPR effect which is similar to those of precious metals, and attracted extensive attention owing to its especial properties including narrow band gap, high carrier motility, and low effective mass [23–25]. In previous reports, Bi/BiPO4 was prepared by using NaBH4 as reducting agent to produce Bi [26]. This method is feasible but the reaction is too intense. Compared to other reducer (KBH4, NaBH4, or ethylene diamine), ethylene glycol (EG) is a mild reagent. Huang et al. [27] used EG to reduce Bi3+ to metallic Bi and fabricated Bi/Bi2WO6 heterojunction that exhibited improved photocatalytic activity.
Herein, based on merits and shortcomings of Bi2O2SiO3 and Bi2WO6 photocatalysts, Bi2O2SiO3/Bi2WO6 composites were prepared by hydrothermal method, and then, metallic Bi was deposited on the surfaces of the Bi2O2SiO3/Bi2WO6 structure by EG reduction due to mild reducibility. And Bi/Bi2O2SiO3/Bi2WO6 composites exhibited the improved activity in photodegradation of RhB and CIP under visible light. The chemical properties and the photocatalysis mechanism of Bi/Bi2O2SiO3/Bi2WO6 were also investigated. It is postulated that the enhanced photocatalytic activity of Bi/Bi2O2SiO3/Bi2WO6 composites should be attributed to the introduction of Bi2O2SiO3 and Bi.
EXPERIMENTAL
Synthesis of the Bi2O2SiO3/Bi2WO6 Photocatalysts
The preparation of Bi2O2SiO3 was reported in [24]. For the preparation of Bi2O2SiO3/Bi2WO6 composites, 0.2425 g Bi(NO3)3 · 5H2O was dissolved in aqueous solution of nitric acid. Meanwhile, 0.0824 g Na2WO4 · 2H2O was dissolved in 10 mL distilled water under constant stirring. Afterward, the Na2WO4 aqueous solution was added to Bi(NO3)3 solution slowly, and stirred for 0.5 h. Then, 0.0346 g Bi2O2SiO3 powders were added to the mixture and stirred further. Finally, the precursor solution was transferred into a 45 mL Teflon-lined autoclave, heated at 160°C for 24 h. The precipitates were washed with deionized water and ethanol for several times, and dried at 80°C. The sample denoted as SW‑20 when the mass ratio of Bi2O2SiO3/Bi2WO6 was 0.2 : 1. According to different mass ratio, other samples were denoted as SW-10 and SW-25, respectively. Bi2WO6 was prepared by the same methods without Bi2O2SiO3.
Synthesis of the Bi/Bi2O2SiO3/Bi2WO6 Photocatalysts
Bi/Bi2O2SiO3/Bi2WO6 heterostructure were prepared by using EG as solvent and reducing agent under solvothermal conditions. Typically, 0.01 g Bi(NO3)3 · 5H2O was dispersed in 80 mL of EG solution. Then, 0.10 g Bi2O2SiO3/Bi2WO6 composites (SW-20) sample was added into the above solution and stirred for 1 h. After that, the suspension was transferred into a 100 mL Teflon-lined stainless steel autoclave and kept at 180°C for 10 h. After cooling, the product was washed repeatedly by distilled water and ethanol, and dried at 80°C. By controlling the mass ratio of Bi(NO3)3 · 5H2O and Bi2O2SiO3/Bi2WO6 composites in the solution were 5, 10, and 15%, the corresponding samples were denoted as BSW-5, BSW-10, and BSW-15, respectively.
Characterization
The crystal phases of the samples were analyzed by X-ray diffraction (XRD, Rigaku Ultima). The surface morphology of the products were analyzed by a Quanta 400 F scanning electron microscopy (SEM). Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HR-TEM) images of the samples were obtained on a HITACHI HT770 transmission electron microscope. Fourier transform infrared spectra (FT-IR) were recorded in KBr pellets with a Nicolet 5700 spectro-meter. UV‒Vis diffuse refectance spectra (DRS) were measured on a UV-2500 UV‒Vis spectrophotometer (Shimadzu, Japan). The total organic carbon (TOC) was measured on a TOC analyzer (TOC-L CPN). The photocurrent and electrochemical impedance spectra (EIS) of the samples were measured out on an electrochemical workstation (CHI 660).
Photocatalytic Activity Measurements
The as-prepared catalyst (20 mg) was added into 50 mL of RhB or CIP aqueous solution (both 10 mg/L) in quartz photochemical reactor and stirred in dark to establish the adsorption equilibrium. Then, the solution was irradiated with a 300 W Xe lamp with a cutoff filter (λ ≥ 420 nm). The reaction mixture was periodically sampled; the samples were centrifuged, extracted and analyzed using UV‒Vis spectrophotometer (UV-3010, Hitachi). The degradation efficiency was calculated as follows:
where C0 is the absorbance of original RhB or CIP solution and C is the absorbance of the RhB or CIP solution after irradiation. The photocatalytic process could be expressed as the following equation:
where k is the apparent pseudo-first-order rate constant. Moreover, the recycling experiments were performed for five recycles to test the durability. After each cycle, the photocatalyst was centrifuged and used directly for the next test.
RESULTS AND DISCUSSION
XRD Analysis
The phase composition of the as-prepared samples was determined by XRD. As shown in Fig. 1, the diffraction peaks of pure Bi2WO6 at 2θ = 28.3°, 32.9°, 47.2°, 56.0°, and 58.5°, were assigned to the (131), (002), (202), (133), and (262) reflection of Bi2WO6 (JCPDS no. 39-0256), respectively. Sharp and intense peaks indicate very high crystallinity [28]. For Bi2O2SiO3, all diffraction peaks could be matched with the orthorhombic phase of Bi2O2SiO3 (JCPDS no. 75-1483) [29]. As for the Bi/Bi2O2SiO3/ Bi2WO6 composites (BSW-10), the weak diffraction peaks at 33.71° was observed, which can be assigned to (002) crystal phases of Bi2O2SiO3 (JCPDS no. 75-1483). Moreover, there is no diffraction peaks for Bi in the composites of BSW-10, which might be due to the weak reducing ability of EG and higher dispersion of composites [30, 31].
XPS Analysis
The XPS survey spectra in Fig. 2a shows the presence of Bi, Si, W, O, C (C comes from reference sample) in BSW-10. Figure 2b features the two strong peaks centered at 164.1 and 158.8 eV are assigned to Bi 4f5/2 and Bi 4f7/2, respectively, which are the features of Bi3+ species of Bi2WO6 [27, 32]. Additionally, the shoulder bands with weak located at 161.8 and 156.6 eV confirms the generation of metallic Bi. As is shown in Fig. 2c, the two peaks at 37.2 and 35.0 eV are assigned to W 4f5/2 and W 4f7/2, which are feature of [WO4]2– [33, 34]. The O 1s profiles in Fig. 2d can be fitted into three peaks, positioned at 529.5, 530.3 and 531.1 eV. The sub-band centered at 529.5 eV is characteristic of lattice oxygen species, such as [Bi2O2]2+ and [WO4]2– layers, and other two peaks around 530.3 and 531.1 eV are assigned to the hydroxyl-like group and the chemically adsorbed water, respectively [35].
SEM and TEM Analysis
The morphology of the as-prepared samples was characterized by SEM and TEM. Figure 3a shows that the pure Bi2O2SiO3 has rod-like structure with the length of rods are 5–10 μm. As shown in Fig. 3b, Bi2WO6 sample consists of flower-like microspheres (~4 μm) self-assembled in nanosheets. It should be noted that the morphology of the BSW-10 composite is similar to that of pure Bi2O2SiO3, with the Bi2WO6 nanosheets focus on the surface of Bi2O2SiO3 rod-like structure and the length of BSW-10 composites about 20 μm (Fig. 3c). To further confirm the composition of composite, the BSW-10 sample was analyzed by TEM and HRTEM. As can be seen from Fig. 4a, there are some irregular spheres on the surface and spheres diameter is about 10–15 nm, which may be metal Bi. Figure 4b shows HRTEM image of BSW-10. By measuring the lattice fringes, the interplanar distance of 0.2717 and 0.3179 nm, which correspond to the (002) and (131) crystal plane of Bi2WO6, respectively. The resolved interplanar distances of 0.2656 and 0.2894 nm are corresponding to (002) plane of Bi2O2SiO3 and (211) plane of Bi (JCPDS no. 51-0765). These results further indicate that the successful fabrication of Bi/Bi2O2SiO3/Bi2WO6 heterojunction, which are consistent with XRD and XPS data.
UV–Vis DRS Spectra
The optical absorption properties of the prepared material were evaluated by DRS (Fig. 5). Compared to Bi2O2SiO3 and Bi2WO6, the composites of BSW-10 showed enhanced visible light absorption (Fig. 5a). Figure 5b shows the plot of (αhν)2/n vs. hν (n = 4 for Bi2O2SiO3 and n = 1 for Bi2WO6), from which the band gap of the samples can be estimated, and the Eg of Bi2O2SiO3, Bi2WO6, BSW-10 are 3.23, 2.90, and 2.83 eV, respectively. Obviously, the light absorption of the Bi/Bi2O2SiO3/ Bi2WO6 heterojunction is broadened the visible light range. The valence band (VB) and the conduction band (CB) potentials can be calculated by the empirical formula:
where X is the absolute electronegativity of the semiconductor, being 6.474 and 5.55 eV for Bi2O2SiO3 and Bi2WO6, respectively [36, 37], EVB and ECB are the VB and CB potentials, respectively, and Ee is the energy of free electrons on the hydrogen scale (4.5 eV vs. NHE). The EVB and ECB of Bi2O2SiO3 were calculated to be 3.62 and 0.39 eV, respectively. For Bi2WO6, the EVB and ECB are 2.5 and –0.4 eV, respectively.
Photocatalytic Activity
In order to study the photocatalytic performance of the samples, the experiments on the photodegradation of RhB as well as CIP were carried out under visible light irradiation. First, compounding Bi2O2SiO3 with Bi2WO6, the photocatalytic activity of RhB on the Bi2O2SiO3/Bi2WO6 composites is shown in Fig. 6a. When the mass ratio of Bi2O2SiO3 reaches 20%, the SW-20 sample’s degradation efficiency of RhB is up to 83.3% under visible light irradiation after 40 min. After the introduction of Bi, BSW-10 sample shows highest activity under visible light irradiation degradation of 93.4% for 40 min. To have a better understanding of the reaction rate, the corresponding first-order kinetics plots were fitted and shown in Fig. 6b [38]. The accuracy of k and R2 parameter were given in Table 1. It is seen that the kinetic plots of the RhB degradation exhibit good linear variation with irradiation time, and the correlation coefficients for the regression lines is larger than 0.9 (i.e., R2 > 0.9).
(Color online) Photocatalytic degradation efficiencies of RhB over different samples (a), first-order kinetics plots for the photocatalytic degradation of RhB under the irradiation of visible light (b), the TOC removal efficiency over different catalysts (c), and photocatalytic degradation efficiencies of CIP under visible light irradiation (d).
It is well known that the mineralization is the final goal in pollutant treatment, and TOC value is usually used as an important index for the mineralization of organic species. The mineralization of RhB was investigated by loading 20 mg catalysts in 50 mL RhB aqueous solution (10 mg L–1) under visible-light irradiation, and TOC value at before and after reaction was recorded. The TOC removal was shown in Fig. 6c, in which the red color marked as the initial TOC value of RhB, and the gray part marked as TOC value after 40 min irradiation. Obviously, with the BSW-10 sample, the final TOC concentration is minimum. This result demonstrates that Bi/Bi2O2SiO3/Bi2WO6 structures can efficiently degrade and mineralize organic pollutants under visible-light irradiation.
To eliminate the photosensitization in the RhB photocatalytic decomposition process, colorless CIP was selected as a model pollutant, as shown in Fig. 6d. This result is similar to the degradation of RhB solution, BSW-10 has the highest photocatalytic activity, the degradation rate of CIP is 69% after visible light irradiation for 80 min.
The stability of Bi/Bi2O2SiO3/Bi2WO6 composites was also studied through the degradation of RhB under visible light irradiation. As displayed in Fig. 7a, after five cycles, the BSW-10 sample still remains high photocatalytic performance and the photocatalytic activity of degradation of RhB is 87.36%, which indicates the Bi/Bi2O2SiO3/Bi2WO6 composites have high stability and reusability in the photocatalytic reaction. Figure 7b shows the FT-IR pattern before and after cycles of BSW-10. The weak peaks located around 1029 cm–1 was decline due to the low content of Bi2O2SiO3 and there is no obvious change in other places, confirming the recyclability of the BSW-10 catalyst.
Photocatalytic Mechanism Analysis
The separation of charges makes a difference in the photodegradation process which can be measured by EIS and the photocurrent. A higher mobility of photoexcited carriers will cause smaller EIS arc radius. Furthermore, higher photocurrent intensity means more electron-hole pairs are generated [39, 40]. As shown in Fig. 8a, the diameter of Nyquist circle of BSW-10 features is smallest radius among all samples, demonstrating the fastest charge transfer property of BSW-10. From Fig. 8b, BSW-10 exhibited the higher photocurrent density in comparison with Bi2WO6, which indicates that BSW-10 generates more electron-hole pairs with excellent separation efficiency under visible light.
It is important to detect the main reactive species in the photocatalytic process to reveal the photocatalytic mechanism. Benzoquinone (BQ), ethylenediaminetetraacetic acid disodium salt (EDTA-2Na), and tert-butanol (TBA) were added to act as the scavengers for superoxide radical (\({}^{\bullet} {\text{O}}_{2}^{ - }\)), holes (h+), and hydroxyl radical (\({}^{\bullet} {\text{OH}}\)), respectively. As shown in Fig. 9, under the visible light irradiation of the BSW‑10 sample, the degradation efficiency of RhB is significantly inhibited by adding EDTA-2Na and BQ scavengers, implying that h+ and \({}^{\bullet} {\text{O}}_{2}^{ - }\) radicals play major roles in the catalytic processes and the addition of TBA shows a weaker influence on the photocatalytic degradation. In the presence of BQ, there was large fluctuation, which may attributed to the adsorption of BQ. This result clearly demonstrates that h+ plays more vital roles than \({}^{\bullet} {\text{O}}_{2}^{ - }\) and •OH in the photodegradation process of RhB over BSW-10 sample.
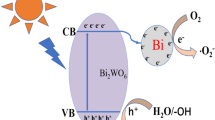
According to the above systematic analysis, a mechanism for the improved photocatalytic activity of Bi/Bi2O2SiO3/Bi2WO6 is proposed, as illustrated in Fig. 10. According to literature [27], the Fermi level (vs. NHE) of Bi can be estimated to be about –0.17 eV and the CB potential of Bi2WO6 is –0.4 eV. Comparing with the Fermi level of metal Bi, the CB of Bi2WO6 is more negative. Due to excellent chemical properties, Bi2WO6 could be excited under visible light irradiation to the photogenerated electrons in the CB and holes in the VB, respectively. Then, the electrons could transfer from CB of Bi2WO6 to Bi metal. Furthermore, the Fermi level of Bi is higher than the CB of Bi2O2SiO3 (+0.39 eV), and the photogenerated electrons in Bi were therefore transferred to Bi2O2SiO3. Compared with the standard redox potential of \({}^{\bullet} {\text{OH}}\)/H2O (+2.8 eV) [41, 42] the VB of Bi2WO6 is not positive enough to generate \({}^{\bullet} {\text{OH}}\). Due to the redox potential of O2/\({}^{\bullet} {\text{O}}_{2}^{ - }\) (–0.33 eV) [43] is more positive than the CB of Bi2WO6, the separated electrons will react with the O2 and reduce it to \({}^{\bullet} {\text{O}}_{2}^{ - }\). Finally, h+ and \({}^{\bullet} {\text{O}}_{2}^{ - }\) degrade pollutants. Therefore, the enhanced photocatalytic performance was ascribed to the formation of Bi/Bi2O2SiO3/Bi2WO6 heterojunction improving the separation of electron-hole pairs.
CONCLUSIONS
In summary, Bi/Bi2O2SiO3/Bi2WO6 composites photocatalyst was prepared by reduction with EG. The photocatalytic activity of Bi/Bi2O2SiO3/Bi2WO6 is evaluated by the photodegradation of RhB and CIP under visible light irradiation. The improved photocatalytic activity can be ascribed to the introduction of Bi2O2SiO3 and the SPR effect of metallic Bi, where the SPR effect of the introduced Bi not only significantly enhances the light absorption, but also leads to efficient separation and transferring of photogenerated charge carriers. This study confirms that connecting the narrow band gap with wide band gap semiconductors, and then, deposition of metallic Bi using EG as a reducing agent could provide a great idea for future photodegradation processes.
REFERENCES
R. Moradi, M. Hamidvand, and A. Ganjali, Russ. J. Phys. Chem. A 93, 1133 (2019).
Y. Huang, K. Yao, M, Xiang, M. Jia, and A. Zhang, Russ. J. Phys. Chem. A 93, 1182 (2019).
Y. Chen, J. Li, and Y. Liang, Russ. J. Phys. Chem. A 93, 1406 (2019).
C. Zou, Z. Yang, M. Liang, Y. He, Y Yun, and S. Yang, Nano 13, 1850070 (2018).
S. Zhu, C. Yang, F. Li, T. Li, M. Zhang, and W. Cao, Mol. Catal. 435, 33 (2017).
Z. Yang, L. Chen, Y. Yang, J. Wang, Y. Huang, X. Liu, and S. Yang, Semicond. Sci. Technol. 32, 065008 (2017).
X. Xiao, J. Wei, Y. Yang, R. Xiong, C. Pan, and J. Shi, ACS Sustainable Chem. Eng. 4, 3017 (2016).
L. Yao, W. Wang, Y. Liang, J. Fu, and H. Shi, Appl. Surf. Sci. 469, 829 (2019).
Y. Zhu, Y. Wang, Q. Ling, and Y. Zhu, Appl. Catal., B 200, 222 (2017).
F. Xu, C. Xu, H. Chen, D. Wu, Z. Gao, X. Ma, Q. Zhang, and K. Jiang, J. Alloys Compd. 780, 634 (2019).
X. Ren, K. Wu, Z. Qin, X. Zhao, and H. Yang, J. Alloys Compd. 788, 102 (2019).
B. Li, C. Lai, G. Zeng, L. Qin, H. Yi, D. Huang, C. Zhou, X. Liu, M. Cheng, P. Xu, F. Huang, and S. Liu, ACS Appl. Mater. Interfaces 10, 18824 (2018).
L. Ji, L. Lin, D. Yu, P. Gao, and B. Liu, Mater. Lett. 220, 94 (2018).
Q. Yang, M. Luo, K. Liu, H. Cao, and H. Yan, Chem. Commun. 55, 5728 (2019).
D. Liu, W. Yao, J. Wang, Y. Liu, M. Zhang, and Y. Zhu, Appl. Catal., B 172, 100 (2015).
D. Liu, J. Wang, M. Zhang, Y. Liu, and Y. Zhu, Nanoscale 6, 15222 (2014).
D. Liu, W. Cai, Y. Wang, and Y. Zhu, Appl. Catal., B 236, 205 (2018).
Z. Wan, and G. Zhang, J. Mater. Chem. A 3, 16737 (2015).
C. Yang, W. Lee, H. Lin, Y. Dai, H. Chi, and C. Chen, RSC Adv. 6, 40664 (2016).
H. Lu, Q. Hao, T. Chen, L. Zhang, D. Chen, C. Ma, W. Yao, and Y. Zhu, Appl. Catal., B 237, 59 (2018).
X. Hu, J. Tian, Y. Xue, Y. Li, and H. Cui, ChemCatChem 9, 1511 (2017).
J. Jia, X. Du, E. Liu, J. Wan, C. Pan, Y. Ma, X. Hu, and J. Fan, J. Phys. D: Appl. Phys. 50, 145103 (2017).
F. Yang, X. Zhu, J. Fang, D. Chen, W. Feng, and Z. Fang, Ceram. Int. 44, 6918 (2018).
X. Li, W. Zhang, J. Li, G. Jiang, Y. Zhou, S. Lee, and F. Dong, Appl. Catal., B 241, 187 (2019).
S. Zhao, Z. Dai, W. Guo, F. Chen, Y. Liu, and R. Chen, Appl. Catal., B 244, 206 (2019).
J. Li, W. Zhang, M. Ran, Y. Sun, H. Huang and F. Dong, Appl. Catal., B 243, 313 (2019).
Y. Huang, S. Kang, Y. Yang, H. Qin, Z. Ni, S. Yang, and X. Li, Appl. Catal., B 196, 89 (2016).
M. Arif, M. Zhang, Y. Luo, H. Yin, and X. Liu, J. Ind. Eng. Chem. 69, 345 (2019).
J. Di, J. Xia, Y. Huang, M. Ji, W. Fan, Z. Chen, and H. Li, Chem. Eng. J. 302, 334 (2016).
X. Liu, L. Pan, T. Lv, and Z. Sun, J. Collid Interface Sci. 394, 441 (2013).
F. Tian, H. Zhao, G. Li, Z. Dai, Y. Liu, and R. Chen, ChemSusChem 9, 1579 (2016).
D. Wang, L. Guo, Y. Zhen, L. Yue, G. Xue, and F. Fu, J. Mater. Chem. A 2, 11716 (2014).
J. Yang, X. Wang, X. Zhao, J. Dai, and S. Mo, J. Phys. Chem. C 119, 3068 (2015).
Y. Peng, M. Yan, Q. Chen, C. Fan, and H. Zhou, J. Mater. Chem. A 2, 8517 (2014).
C. Wu, H. Chang, and J. Chern, J. Hazard. Mater. 137, 336 (2006).
J. Liu, Y. Li, Z. Li, J. Ke, and H. Xiao, Catal. Today 314, 2 (2018).
X. Liu, W. Wang, Y. Liu, B. Huang, Y. Dai, X. Qin, and X. Zhang, RSC Adv. 5, 55957 (2015).
H. Dong, G. Chen, J. Sun, Y. Feng, C. Li, and G. Xiong, Dalton Trans. 43, 7282 (2014).
C. Wang, X. Zhang, Y. Zhang, J. Chen, G. Huang, J. Jiang, W. Wang, and H. Yu, Appl. Catal., B 237, 464 (2018).
S. Xiao, Y. Li, J. Hu, H. Li, X. Zhang, L. Liu, and J. Lian, CrystEngComm 17, 3809 (2015).
J. Wang, L. Tang, G. Zeng, Y. Liu, Y. Zhou, Y. Deng, J. Wang, and B. Peng, ACS Sustainable Chem. Eng. 5, 1062 (2017).
S. Kumar, A. Baruah, S. Tonda, B. Kumar, V. Shanker, and B. Sreedhar, Nanoscale 6, 4830 (2014).
S. Wang, X. Yang, X. Zhang, X. Ding, Z. Yang, K. Dai, and H. Chen, Appl. Surf. Sci. 391, 194 (2017).
ACKNOWLEDGMENTS
This work was kindly supported by the National Natural Science Foundation of China (21171053), Hubei Province Undergraduate Training Programs for Innovation and Entrepreneurship (201810513073), Graduate Student Innovation Research Foundation of Hubei Normal University (2018039) and Institute for Advanced Materials of Hubei Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhi Zhang, Zou, C., Zhou, J. et al. Synthesis of Bi/Bi2O2SiO3/Bi2WO6 Composites with Enhanced Visible Light Activity in Photocatalytic Degradation of Organic Compounds. Russ. J. Phys. Chem. 94, 1254–1261 (2020). https://doi.org/10.1134/S0036024420060369
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024420060369