Abstract
Values of specific electric conductivity are determined for nitrogen-doped carbon nanotubes (N‑CNTs) and N-CNTs oxidized with 68 wt % HNO3. It is established that the electrical conductivity of the material falls considerably along with nitrogen content in the tubular structure of the N-CNTs and an increase in the number of oxygen-containing groups on their surfaces due to a rise in the concentration of defects in their structure. The electrical conductivity of all types of samples rises along with pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Carbon nanotubes (CNTs) and their nitrogen-doped modifications are promising materials for a variety of applications, due to their combination of high electrical conductivity and thermal and mechanical strength [1]. They are used as adsorbents [2], materials for the electrodes of supercapacitors [3], carriers of catalysts [4], and materials for nanoelectronics [5]. The heterosubstitution by nitrogen atoms of graphene and graphite in the structure of CNTs is currently considered as one of the most effective ways of altering their conductive properties [6–8]. Depending on such characteristics of N-CNT structure as nitrogen content and geometric dimensions, N-CNTs can exhibit either semiconductor conductivity [9] and metallic, as was confirmed theoretically in [10–13]. The electrical conductivity of nitrogen-doped carbon nanomaterials (N-CNM) depends on the number of nitrogen atoms and the type of their coordination (graphitic, or quaternary nitrogen, pyrrolic nitrogen, and pyridinic nitrogen) in the structure of the considered material. An increase in the nitrogen content normally lowers the value of this parameter. For example, the electrical conductivity of N-doped single-walled CNTs falls from 1800 to 1000 Ω−1 cm–1 when their nitrogen content is raised from 0.4 to 1.6%, while N‑CNTs exhibit conductivities ranging from 325 to 60 Ω−1 cm−1 when their nitrogen content is raised from 4 to 7.4% [15]. The presence of graphitic nitrogen in the structure results in the n-type conductivity characteristic of these materials [16], and raising the nitrogen fraction improves their electrical conductivity [17]. At the same time, raising the number of pyridinic nitrogen atoms lowers this parameter, due to an increase in the defectiveness of the N-CNT structure [18].
The final result is affected by means of doping, i.e., the post-doping of CNT surfaces using ammonia or the bulk doping of the entire CNT volume directly in obtaining N-CNMs [19, 20]. It is known that the electrical resistance of CNTs falls from 0.5 to 0.42 Ω after doping of only their surface layers (nitrogen content, 8.6%) [21]. The electrical conductivity of the material is also affected by the level of functionalization, the length of the CNTs [15, 22], and the presence of impurities [23]. For example, the electrical conductivity of soot grows along with the concentration of oxygen- and sulfur-containing groups on its surface [24], but it falls after the treatment of CNTs with nitric acid [22].
The aim of this work was to perform a systematic study of the electrical conductivity of N-CNTs, depending on their morphology, nitrogen content, and the type of nitrogen atoms in their structure, along with their level of surface functionalization by oxygen-containing groups. Most of our efforts were devoted to effect of the pressure and density of oxidized N-CNTs on their electrical conductivity.
EXPERIMENTAL
N-Doped CNTs were obtained via the pyrolytic decomposition of acetonitrile at 750°C for 5 h in the presence of Co,Mo@MgO catalyst, according to [25]. The produced N-CNTs were purified by boiling in a 30 wt % HCl solution (Megakhim, Russia), followed by washing with distilled water until a neutral pH value was obtained and drying for 24 h at 120°C. For purposes of surface functionalization, oxidative treatment of the N-CNTs was performed using a 68 wt % HNO3 solution (Reakhim, Russia) with boiling under reflux for 0.5–12 h. Depending on the duration of oxidation, the samples were labeled N‑CNT_x, where x = 0.5, 1, 1.5, 2, 4, 6, and 12, according to the duration of oxidative treatment in hours.
The samples of N-CNTs were studied via TEM on a JEOL JEM 2100 F-Cs electron microscope (JEOL Ltd., Japan) equipped with an analyzer of electron energy loss spectra and a corrector of spherical aberrations. The technique of the sample preparation for analysis included dispersing of a weighed portion (1 mg) of a sample in water with 15 min of ultrasound exposure. A drop of the resulting suspension was then applied onto a copper mesh preliminarily coated with a polymer layer.
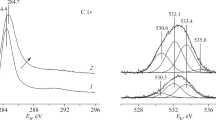
The nitrogen content and surface functional groups were analyzed by means of X-ray photoelectron spectroscopy (XPS) using an Axis Ultra DLD spectrometer (Kratos Analytical Ltd., United Kingdom). The obtained spectra were decomposed with the CasaXPS and Unifit 2006 software packages.
The electrical conductivity of the material was measured using the two-probe approach with a Hounsfield H5K-S testing machine to create pressures of up to 3 MPa. The current contacts were connected to a GPD-73303D DC source at a room temperature. The voltage across the potential contacts was measured with a GDM-78255A portable voltmeter. To exclude the thermoelectromotive force, all measurements were made for two opposite directions of current. The specific resistance was calculated according to the formula
where U is voltage, V; I is current strength, A; d is cell diameter, m; and h is sample thickness, m.
RESULTS AND DISCUSSION
The morphology of N-CNTs before (Figs. 1a, 1b) and after (Figs. 1c–1g) treatment with nitric acid was studied by HRTEM. The N-CNTs had a structure characterized by a conical arrangement of graphene layers, multiple intrachannel bottlenecks, and thus defects and bends. It was apparently a consequence of the drop in the tension of the structure that occurred after the incorporation of nitrogen atoms [25] with coordination different from that of the sp2 hybrid carbon atoms in the graphene planes and the falling internal energy of the system [26]. The structure of graphene layers was destroyed upon increasing the duration of N-CNT oxidation by nitric acid, as was especially evident after 4 h of treatment in HNO3 (Figs. 1d–1g). The tubular structure of the N-CNTs after 6 h of treatment with nitric acid (N-CNT_6) was almost completely lost (Fig. 1f), while N-CNT_12 contained a large amount of unstructured carbon after 12 h (Fig. 1g).
Upon the oxidation of N-CNTs, the total nitrogen content in the structure fell from 2.77 at % (N-CNTs before treatment) to the minimum value of 0.52 at % for N-CNT_12 (Fig. 2a), as was confirmed by XPS spectra described in detail in [25]. Electrical conductivity σ observed for the samples was nonlinear, depending on the total nitrogen content. Three areas are conventionally distinguished. The maximum value of electrical conductivity, σ = 46.7 ± 0.1 Ω−1 m−1, was exhibited by N-CNTs with a nitrogen content of 2.77 at % (before oxidation in nitric acid). Oxidation reduced the content of nitrogen in the N-CNT structure, but samples N-CNT_1.5, N-CNT_2, and N‑CNT_4 (nitrogen content 1.80, 1.40, and 0.92 at %, respectively) had close values: σ = 6.3 ± 0.1, 6.5 ± 0.2, and 6.3 ± 0.2 Ω−1 m−1, respectively. A further drop in electrical conductivity to 3.4 ± 0.1 Ω−1 m−1 for N‑CNT_12 was observed during the subsequent oxidation of the samples.
Electrical conductivity (σ) of the oxidized N-CNT samples for different durations of oxidative treatment depending on (a) the nitrogen content and (b) the fraction of nitrogen atoms in different states. (1–3) correspond to pyridinic, graphitic (quaternary), and pyrrolic nitrogen atoms, respectively.
The physicochemical properties of nitrogen-doped carbon materials depend strongly on the number and ratio of nitrogen atoms in such different states as pyridinic, graphitic (quaternary), and pyrrolic [18, 26, 27]. In this work, we established that the electrical conductivity changed symbatically (Figs. 2a, 2b), depending on the total nitrogen content and the amount of nitrogen in the graphitic state. The correlations between the content of the pyrrolic and pyridinic nitrogen atoms and the electrical conductivity were almost linear (Fig. 2b). Such complicated dependences of the electrical conductivity in N-CNTs on the nitrogen content were observed in [15, 18, 28, 29]. The authors of [18] found that graphitic nitrogen affected the value of σ most strongly, while increasing its content rose the value of σ. The effect of pyridinic nitrogen was demonstrated in [30]. In this work, the ratio between the contents of graphitic and pyridinic nitrogen in the studied samples changed nonlinearly. This ratio was 1.6 for sample N‑CNT_0.5 and 2.4 for N-CNT_1.5. It was identical for samples N-CNT_2 and N-CNT_4 at 1.6, and rose to 2 and 3.8 for N-CNT_6 and N‑CNT_12, respectively. The change in the content of all types of nitrogen atoms and their ratio increased the disorder (imperfection) in the structure, as was confirmed via TEM (Fig. 1) and Raman spectroscopy [25]. The electrical conductivity thus apparently depended on all these factors.
The electrical conductivity depended not only on the amount of nitrogen in the structure of the samples, but also on the amount of such adsorbed gases as O2, N2, and H2 [31]; and on the number of surface groups that participated in charge transfer. The conductive properties of CNMs normally deteriorate along with their functionalization [22, 23]. According to XPS data, the oxidation of N-CNTs resulted in the growth of the concentration of oxygen-containing surface groups. Their content varied in a complicated manner, reaching the highest value of 8.07 at % at a 1.5 h duration of treatment. We may assume that the destruction of the CNT structure during oxidation occurs mainly at the sites of the incorporation of nitrogen atoms, while the content of nitrogen falls.
An increase in pressure raises the conductivity of powders of different composition considerably [32] along with that of CNMs, especially carbon black [33], graphite [34], and CNTs [35]. However, the electrical conductivity corresponding to an individual particle cannot be achieved even at high and maximum degrees of compaction (100% of the theoretically calculated material density), since the contact effect cannot be nullified completely. It has been established that the bulk density of oxidized N-CNTs grows along with external pressure and the duration of oxidation (Fig. 3). It was shown in [25] that the treatment of N-CNTs with nitric acid was accompanied by three main textural changes: compaction of the material, the opening of the ends of the tubes, and the degradation of their structure [25]. The bulk density of our samples without external pressure grew along with the duration of oxidation, from 0.31 to 1.05 g cm−3. The maximum density measured at 2.7 MPa was 1.16 g cm−3 for N-CNT_0.5 (Fig. 3). This value is much lower than those inherent in CNTs, amorphous carbon, or graphite, whose experimentally determined densities are 1.74, 1.84–2.06, and 1.78 g cm–3, respectively [36, 37].
The electrical conductivity of oxidized N‑CNTs grew considerably upon raising both the pressure and density of the samples (Fig. 4). The conductivity curves for N-CNT_1.5 and N-CNT_2 are close, due apparently to the morphology of these samples being similar. A maximum electrical conductivity of 138.9 Ω−1 m−1 was found for sample N-CNT_0.5 at a pressure of ~3 MPa. Such an increase in electrical conductivity with rising sample density was described in [38] for CNTs and carbon nanofibers. The values of σ for those samples were 900 and 450 Ω−1 m−1, respectively.
CONCLUSIONS
Treatment of N-CNTs with nitric acid results in surface functionalization with oxygen-containing groups with simultaneous destruction of the structure, a drop in the nitrogen content, and a reduction in electrical conductivity σ. Despite a rise in the density of samples when the duration of their oxidation was increased, their electrical conductivity fell. This confirms that the nature of a sample itself (its composition, morphology, and surface) has a greater effect on the conductive properties of the considered materials than their density. It was shown that the electrical conductivity grew along with pressure when making measurements.
REFERENCES
R. H. Baughman, A. A. Zakhidov, and W. A. de Heer, Science (Washington, D.C., U. S.) 297, 787 (2002).
A. Purceno, B. Machado, A. Teixeira, et al., Nanoscale 7, 294 (2015).
Z. Liu, Y. Tao, X. Song, et al., RSC Adv. 7, 6664 (2017).
S. Savilov, A. Ivanov, E. Suslova, et al., Adv. Mater. Res. 364, 444 (2012).
O. Podyacheva and Z. Ismagilov, Catal. Today 249, 12 (2015).
J. Pels, F. Kapteijn, J. Moulijin, et al., Carbon 33, 1641 (1995).
R. Czerw, M. Terrones, J. Charlier, et al., Nano Lett. 1, 457 (2001).
Y. Wang, Y. Shao, D. Matson, et al., ACS Nano 4, 1790 (2010).
D. Kim, C. Lin, T. Mihalisin, et al., Chem. Mater. 3, 686 (1991).
J. Wilder, L. Venema, A. Rinzler, et al., Nature (London, U.K.) 391, 59 (1998).
M. Mattesini, S. Matar, and J. Etourneau, J. Mater. Chem. 10, 709 (2000).
Y. Huang, R. Gao, and R. Liu, Synth. Met. 113, 251 (2000).
A. Shalagina, Z. Ismagilov, O. Podyacheva, et al., Carbon 45, 1808 (2007).
E. Ibrahim, V. Khavrus, A. Leonhardt, et al., Diamond Relat. Mater. 19, 1199 (2010).
J. Wiggins-Camacho and K. Stevenson, J. Phys. Chem. C 113, 19082 (2009).
C. Ewels and M. Glerup, J. Nanosci. Nanotechnol. 5, 1345 (2005).
I. Kunadian, S. Lipka, C. Swartz, et al., J. Electrochem. Soc. 156, K110 (2009).
O. Podyacheva, S. Cherepanova, A. Romanenko, et al., Carbon 122, 475 (2017).
R. Taylor and A. Humffray, J. Electroanal. Chem. 64, 63 (1975).
Z. Zhang, D. Tryk, and E. Yeager, Proc. Electrochem. Soc., 84 (1984).
B. An, S. Xu, L. Li, et al., J. Mater. Chem. A 1, 7222 (2013).
I. Mazov, V. Kuznetsov, I. Simonova, et al., Appl. Surf. Sci. 258, 6272 (2012).
Q. Li, Y. Li, X. Zhang, et al., Adv. Mater. 19, 3358 (2007).
D. Pantea, H. Darmstadt, S. Kaliaguine, et al., Carbon 39, 1147 (2001).
E. Arkhipova, S. Ivanov, N. Strokova, et al., Carbon 125, 20 (2017).
E. Suslova, K. Maslakov, S. Savilov, et al., Carbon 102, 506 (2016).
E. Suslova, S. Savilov, J. Ni, et al., Phys. Chem. Chem. Phys. 19, 2269 (2017).
H. Chen, F. Sun, J. Wang, et al., J. Phys. Chem. C 117, 8318 (2013).
Z. Ismagilov, A. Shalagina, O. Podyacheva, et al., Carbon 47, 1922 (2009).
K. Fujisawa, T. Tojo, H. Muramatsu, et al., Nanoscale 3, 4359 (2011).
G. U. Sumanasekera, C. K. W. Adu, B. K. Pradhan, et al., Phys. Rev. B 65, 035408 (2001).
K. Euler, J. Power Sources 3, 117 (1978).
J. Sanchez-Gonzalez, M. Macıas-Garcıa, M. Alexandre-Franco, and V. Gomez-Serrano, Carbon 43, 741 (2005).
S. Marinkovic, C. Suznjevic, and M. Djordjevic, Phys. Status Solid A 4, 743 (1971).
B. Marinho, M. Ghislandi, E. Tkalya, et al., Powder Technol. 221, 351 (2012).
T. Sogabe and T. Matsumoto, J. Eur. Ceram. Soc. 16, 395 (1996).
S. Kim, G. Mulholland, and M. Zachariah, Carbon 47, 1297 (2009).
I. V. Zolotukhin, I. M. Golev, A. E. Markova, S. N. Blinov, D. A. Grishin, and E. G. Rakov, Tech. Phys. Lett. 31, 159 (2005).
Funding
This work was financially supported by the Russian Foundation for Basic Research (project GFEN_a no. 18-53-53032) in the scope of State assignments for research organizations and the Development Program of the Moscow State University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by O. Polyakov
Rights and permissions
About this article
Cite this article
Suslova, E.V., Arkhipova, E.A., Kalashnik, A.V. et al. Effect of the Functionalization of Nitrogen-Doped Carbon Nanotubes on Electrical Conductivity. Russ. J. Phys. Chem. 93, 1952–1956 (2019). https://doi.org/10.1134/S0036024419100303
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419100303