Abstract
The intermolecular interactions in (CX4)2 dimers (X = H, F, Cl, Br, I) were studied by the ab initio methods. The calibration values of the energies and bond lengths of the dimers were obtained by the coupled-cluster method taking into account single and double excitations and the non-iterative correction for triple excitations (CCSD(T)) with Dunning’s basis sets complemented with bond functions centered between the carbon atoms, followed by extrapolation to the infinite basis set limit. An analysis of the constructed PES cross sections of the (CX4)2 dimers allowed us to substantially refine the dissociation energies for (CCl4)2 and (CBr4)2 from previous calculations and to evaluate the dissociation energy of the (CI4)2 dimer. The constructed correlations between the calculated dissociation energies of the (CX4)2 dimers and the polarizabilities of CX4 revealed the critical role of polarizability of individual molecules in the variation of the physical properties of CX4 dimers with X changed from fluorine to iodine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Understanding the intermolecular forces that determine the physicochemical features of halogen-substituted alkanes is important for their applications in many areas from petrochemistry to pharmacology. They are believed to be characterized by specific halogen–halogen interactions. The simplest representatives of systems whose interactions are completely determined by these forces are perhalomethane (CX4)2 dimers (X = F, Cl, Br, I). This series can also include the methane dimer, which can be considered a zeroth compound in the series of haloalkanes. As correct theoretical description of specific van der Waals interactions is a complex problem, this led to inclusion of methane in the test set of molecules for analyzing the quality of the density functionals S22 [1], and the use of high-precision quantum-chemical methods made it possible to estimate the intermolecular interaction energy at 0.51 kcal/mol with an error of less than 1% [2]. This allows these methods to be used to construct the force fields, subsequently using them in modeling both the gas-phase characteristics (virial coefficients, thermodynamic functions) and the condensed phase states (phase transitions, mobility, matrix isolation).
The halogen-substituted systems were not studied very thoroughly, moreover the accuracy of description decreases from fluorine to iodine. The best estimate of the intermolecular interaction energy for the (CF4)2 dimer is 0.74 kcal/mol obtained by the coupled-cluster method including the single and double excitations and the non-iterative correction for triple excitations (CCSD(T)) for the Suzuki basis set [3]. The energy of interaction between the CCl4 molecules was calculated at the level of the second-order Möller–Plesset perturbation theory (MP2) with extrapolation to an infinite basis set and was found to be 3.523 kcal/mol [4]. The (CBr4)2 dimer was studied to even a lesser extent; the interaction energy between the monomers was determined by the MP2 method with the aug(df)-6-311G* basis set, 3.702 kcal/mol [5]. The data on the periodomethane dimer are unavailable in the literature.
The dimers of inert gases can be the analogs of these van der Waals systems. For this case, it was shown [6–8] that the best description is achieved by using the F12-CCSD(T) or CCSD(T) methods with basis sets containing bond functions. This is explained by the fact that the main contribution to the interaction energy is made by the effects caused by the presence of intermolecular electron correlation, which today can be correctly included in calculation for large systems only by the indicated methods [6]. However, these approaches are not equivalent. The explicitly correlated F12-CCSD(T) method most accurately describes the orientation and induction interactions between polar molecules, while the dispersion interactions, characteristic of nonpolar systems including the methane dimer, can be most effectively determined by the CCSD(T) method with addition of bond functions [9].
The goal of this study was to perform high-precision calculations of the structural and energy properties of the (CX4)2 dimers (X = H, F, Cl, Br, I) within the framework of a single method based on the use of the coupled-cluster CCSD(T) method with an additional set of bond functions; verification of the results using the available data (X = H, F); comparison of the obtained data with the data obtained by known lower-level calculations (X = Cl, Br); and verification of the results based on correlation analysis using the known experimental data.
Systems under Study and Physical Quantities
The interaction potentials of the (CX4)2 dimers as functions of the distance r(C–C) are of prime interest. As the interaction energy of van der Waals systems is mainly determined by the polarizabilities of individual molecules, the polarizabilities of X atoms and CX4 molecules were also calculated to establish correlations in the series under consideration. To assess the quality of description, the estimates of the equilibrium distance C–X in the individual CX4 molecules were checked.
Details of Ab initio Simulation
To describe the electron wave function, Dunning’s correlation-consistent basis sets cc-pVNZ and aug-cc-pVNZ (N = D, T, Q) were used. At the preliminary stage, the following ways to describe halogen atoms were tested. For chlorine atoms, both the full-electron (FE) basis sets and the basis sets combined with the effective core potentials (ECPs) 10SDF [10] and 10MWB [11] were used. For bromine atoms, only the effective core potentials 10MDF [12], 28MDF [13], and 28MWB [11] were used. For iodine atoms, the effective core potentials 28MDF [14] and 46MDF [13] were employed; in addition, the aug-cc-pwCVTZ-DK3 basis set [15] was used, which takes into account the scalar relativistic effects.
The polarizabilities were determined by the finite-field method. For halogen atoms in the 2Po state, both components of anisotropic polarizability in the nonrelativistic case were calculated because, according to [16], the isotropic component changes insignificantly when the spin-orbital effects are included in calculation, while the anisotropic component is small. The symmetry of the wave function component for halogen atoms was determined by the orbital population; \({{C}_{{{\text{2}}v}}}\) symmetry was used in the calculation. The equilibrium geometry of CX4 was determined by the gradient optimization method with a threshold value of 1 × 10–6 au. The obtained energies were extrapolated to the complete basis set limit using the two-parameter dependence on the reciprocal maximum orbital moment of the basis to the third power proposed by Helgaker [17].
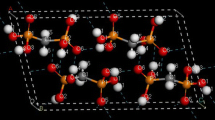
The most time-consuming problem was the construction of the PES cross sections of the (CX4)2 dimers. Preliminary tests showed that all the complexes under investigation had equilibrium geometry of D3d symmetry previously described in the literature (Fig. 1). In the MP2, CCSD, and CCSD(T) methods with the basis sets used, the cross section was constructed only along the C–C distance; the geometry of the CX4 monomers remained rigid. To describe the systems with X = Cl, Br, I, only one version of the effective core potentials was used, which was chosen according to the results of the study of individual molecules.
For detailed description of the intermolecular electron correlation, we used the well-proven technique of adding the basis functions [7, 18, 19] in the middle of the C–C distance. The corresponding [3s3p2d2f1g] basis set was taken from [7]. The obtained interaction energies were extrapolated to the complete basis set limit by a method similar to that used for individual molecules. All the calculations were performed in the Molpro software package [20].
Results of Calculations
The calculation of the components of polarizabilities of the individual X atoms (X = H, F–I) showed that the scatter of values was up to 10% relative to the results obtained by the CCSD(T) method with the aug-cc-pVQZ basis for all the systems already at the level of the Hartree–Fock method. The use of various ECPs did not affect the results. The estimates obtained at the MP2 level using the triply split-valence basis sets gives polarizability estimates with an error of less than 3% in all instances. As the description of van der Waals interactions requires a deeper level of calculation of electron correlation effects, it can be concluded that the errors in the description of individual molecules at this stage will be significantly smaller than the error in the intermolecular interaction energy.
For CX4 molecules, the equilibrium C–X distances were calculated at the level of the CCSD(T) method in the complete basis set limit: 1.30, 1.75, 1.94, and 2.10 Å for fluorine, chlorine, bromine, and iodine, respectively. The average value of polarizability found by the CCSD(T) method with the aug-cc-pVTZ basis set was 18.96, 66.20, 94.06, and 159.46\(a_{0}^{3}\) for CF4, CCl4, CBr4, and CI4, which agrees within 5% with the experimental data for the former three molecules: 19.31 [21], 70.92 [22], and 101.29\(a_{0}^{3}\) [23]. There are no data on the polarizability of periodomethane in the literature.
Based on the calculations of individual atoms and CX4 molecules, it was concluded that the core correlation does not play a significant role in the geometry and polarizability determination for the studied systems. For the chlorine-containing systems, the results obtained for 10 core electrons (with the 1s, 2s, and 2p shells included in the model potential) using ECP and those of full-electron calculations were compared; in the latter case, the results of MP2, CCSD, and CCSD(T) calculations were compared with those obtained with inclusion of excitations from both the core orbitals and only from the valence 3s and 3p shells. Both the geometry and polarizability proved very sensitive to the choice of the method for calculating the electron correlation, while the deviations in polarizability did not exceed 2% when comparing the data obtained using ECP with those of full-electron calculation with excitations from all the core orbitals included in the calculation. A similar conclusion was made for bromine, for which the results obtained using ECP were compared for the cases of 10 (1s, 2s, 2p) and 28 (1s, 2s, 2p, 3s, 3p, 3d) electrons; the error in polarizability was 1.7%. For iodine, the use of the Dirac–Fock pseudopotential, which includes scalar relativistic effects, was validated by comparing the CCSD(T) data for effective core potentials and for all electrons described in the second-order Douglas–Kroll formalism in the aug-cc-pwCVTZ-DK3 basis. The difference in polarizability was 2.1%; the relative deviation of the equilibrium C‒I distance did not exceed 0.2%.
Based on the data obtained, effective core potentials containing the maximum number of core and valence electrons in the model part were chosen for further studies of the dimers: 10SDF, 28MWB, and 46MDF for chlorine-, bromine-, and iodine-substituted methanes.
The most important results of PES cross section calculations along the C–C distance are presented in Tables 1 and 2. In all cases, it is implied that the calculations were performed using additional bond functions placed in the middle of the C–C distance.
The influence of the level of the quantum-chemical description using the methane dimer as an example is illustrated in Fig. 2. Figure 3 shows the PES cross sections obtained in the chosen reference method (CCSD(T)/aug-cc-pVTZ + bf[33221]) for the whole series of studied dimers.
The use of doubly split-valence basis sets leads to a systematic increase in the error from fluorine to iodine. Transition from aug-cc-pVDZ to aug-cc-pVTZ gives the differences between the adjacent PES cross sections of ~1, 1.5, 5, 10, and 20% for X = H, F, Cl, Br, and I, respectively.
The intermolecular electron correlation is the main factor affecting the intermolecular interaction in the (CX4)2 systems. Thus, the CCSD(T) method with the effective core potential that includes all the core and subvalent electrons using the aug-cc-pVTZ basis set complemented with the bond functions [33221] gives the calibration data. The dissociation energy of the perfluoromethane dimer differs from the estimate obtained by the same method but using a substantially poorer basis set by 12% [3]. The MP2 calculated interaction energies of the monomers extrapolated to the complete basis set limit correspond to the dissociation energies 3.523 and 3.702 kcal/mol of perchloromethane and perbromomethane evaluated by the same method [4, 5], but exceed the calibration estimates of CCSD(T) by a factor of 1.6 and 1.3, respectively.
Polarization Correlations
According to the Slater–Kirkwood equation in the interpretation of Mavroyannis [24], the energy of the dispersion interaction between the A and B molecules is determined by the polarizabilities αA and αB of the A and B molecules, the distance Re between them, and the number of electrons NA and NB involved in the van der Waals interaction:
In the case of identical CX4 molecules, the dispersion energy is proportional to the polarizability of CX4 to the power of 1.5. The constructed dependence of \({{D}_{{\text{e}}}}R_{{\text{e}}}^{6}\) on the polarizability of CX4 (X = F, Cl, Br, I) to the power of 1.5 is presented in Fig. 4. It is described by a linear function with a correlation coefficient of 0.9963. From the parameters of the linear dependence it is also possible to obtain an empirical estimate of the number of electrons, which was found ~31. In CX4 (except methane), there are 28 valence electrons from halogen atoms, and one electron can be additionally assigned to each halogen atom due to the formation of a polar covalent bond. Thus, the Slater–Kirkwood equation can be used to describe complex systems, considering only the valence electrons of atoms involved in the van der Waals interaction and applying a correction for additional polarization of the molecule. The correlation of the polarizability of CX4 with the polarizabilities of X, X–, and X2 shown in the diagram (Fig. 5) indicates that the former is determined by the polarizability of the X substituents that is intermediate between the polarizabilities of the X atom and the X– anion.
Thus, a precise approach was developed for calculating the interaction energy in the СX4 dimers (X = H, F, Cl, Br, I). Based on the constructed correlations, it was established that a significant increase in the polarizability of atoms in the fluorine–iodine series is the main factor that determines the drastic change in the properties of substances in the perfluoromethane—periodomethane series. The periodomethane dimer—an unstable chemical compound—was theoretically studied here for the first time. Its dissociation energy was determined by the CCSD(T) method in the complete basis set limit using the 46MDF core pseudopotential (4.14 kcal/mol).
REFERENCES
L. F. Molnar, X. He, B. Wang, and K. M. Merz, Jr., J. Chem. Phys. 131, 0651002 (2009).
A. H. Li and S. D. Chao, J. Mol. Struct: THEOCHEM 897, 90 (2009).
M. J. Biller and S. Mecozzi, Mol. Phys. 110, 377 (2012).
A. H. Te-Li, S.-C. Huang, and S. D. Chao, J. Chem. Phys. 132, 024506 (2010).
R. Mahlanen, J.-P. Jalkanen, and T. A. Pakkanen, J. Chem. Phys. 313, 271 (2005).
S. M. Cybulski and R. R. Toczylowski, J. Chem. Phys. 111, 10520 (1999).
Fu-Ming Tao and Yuh-Kang Pan, J. Chem. Phys. 97, 4989 (1992).
K. Patkowski and K. Szalewicz, J. Chem. Phys. 133, 094304 (2010).
K. Patkowski, J. Chem. Phys. 138, 154101 (2013).
G. Igel-Mann, H. Stoll, and H. Preuss, Mol. Phys. 65, 1321 (1988).
A. Bergner, M. Dolg, W. Küchle, et al., Mol. Phys. 80, 1431 (1993).
K. A. Peterson, D. Figgen, E. Goll, et al., J. Chem. Phys. 119, 11113 (2003).
H. Stoll, B. Metz, and M. Dolg, J. Comput. Chem. 23, 767 (2002).
K. A. Peterson, B. C. Shepler, D. Figgen, and H. Stoll, J. Phys. Chem. A 110, 13877 (2006).
D. H. Bross and K. A. Peterson, Theor. Chem. Acc. 133, 1434 (2013).
T. Fleig and A. J. Sadlej, Phys. Rev. A 65, 032506 (2002).
T. Helgaker, W. Klopper, H. Koch, and J. Noga, J. Chem. Phys. 106, 9639 (1997).
A. A. Buchachenko and N. F. Stepanov, J. Chem. Phys. 106, 10134 (1997).
A. A. Buchachenko, O. Roncero, and N. F. Stepanov, Russ. J. Phys. Chem. A 74, 193 (2000).
H.-J. Werner, P. J. Knowles, G. Knizia, et al., MOLPRO, Version 2010.1, A Package of ab initio Programs (2010).
J. W. Au, G. R. Burton, and C. E. Brion, Chem. Phys. 221, 151 (1997).
T. N. Olney, N. M. Cann, G. Cooper, and C. E. Brion, Chem. Phys. 223, 59 (1997).
E. W. Blanch, R. I. Keir, and G. L. D. Ritchie, J. Phys. Chem. A 106, 4257 (2002).
C. Mavroyannis and M. J. Stephen, Mol. Phys. 5, 629 (1962).
C. Hättig and B. A. Heß, J. Chem. Phys. 108, 3863 (1998).
F. Holka and M. Urban, J. Chem. Phys. 141, 214303 (2014).
G. Maroulis, Mol. Phys. 77, 1085 (1992).
E. F. Archibong and A. J. Thakkar, Chem. Phys. Lett. 201, 485 (1993).
G. Maroulis and A. J. Thakkar, Mol. Phys. 73, 1235 (1991).
M. S. A. FI-Kader and Y. N. Kalugina, Chem. Phys. Lett. 639, 93 (2015).
T. M. Miller and B. Bederson, Adv. At. Mol. Phys. 13, 1 (1978).
M. Gussoni, R. Rui, and G. Zerbi, J. Mol. Struct. 447, 163 (1998).
ACKNOWLEDGMENTS
We are grateful to N.F. Stepanov for useful discussion of the results, to D.A. Shulga for discussing the interaction in halogenated systems, and to T.M. Ro-shchina for discussing the importance of describing the interaction during the adsorption of perfluorinated compounds. This study was financially supported by the Russian Scientific Foundation (grant no. 17-13-01466). The calculations were performed using the computer resources of the Research Computing Center, Moscow State University, and of the Skolkovo Institute of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Rights and permissions
About this article
Cite this article
Rutskoy, B.V., Bezrukov, D.S. Ab Initio Description of the Structure and Interaction Energy of Perhalomethane Dimers. Russ. J. Phys. Chem. 93, 1519–1524 (2019). https://doi.org/10.1134/S0036024419080259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036024419080259