Abstract
The molecular structure of an ethylene glycol–water mixture (EGW) in which EG concentration \({{x}_{{{\text{EG}}}}} = 0{-}1.0\) is analyzed using the concentration dependences of viscosity and specific electrical conductivity at 298 K. Values of excess viscosity, electrical conductivity, and the Grunberg–Nissan parameter are calculated for the mixture. The maximum electrical conductivity is observed when xEG = 0.11 and the EG : water ratio is 1 : 8. The minimum excess viscosity is observed when xEG = 0.46 and the EG : water ratio is 3 : 4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Ethylene glycol (EG, HOCH2CH2OH, ethanediol-1,2) is a simple polar molecule. EG is the simplest dihydric alcohol. It is a hygroscopic liquid completely miscible with water and many other polar solvents. Like water, EG forms a spatial network of hydrogen bonds [1, 2] that largely determines its physical and chemical properties. Being a universal solvent, EG is of great interest in the pharmaceutical and chemical industries.
EG has been studied both theoretically [2–5] and experimentally via dielectric relaxation [6], infrared [7], microwave [8], and NMR spectroscopies [9, 10].
EG–water mixtures enter a glassy state even at relatively low concentrations of EG, preventing crystallization at negative temperatures. EG is therefore an important cryoprotectant [11]. EG may be considered a model system when studying the competition between intramolecular and intermolecular hydrogen bonds.
Due to great practical importance of EG, there have been many works devoted to EG aqueous solutions containing various salts and dissolved gases. They presented data on phase equilibria [12–14] and thermodynamic and transport properties [15, 16], allowing simulation of physicochemical processes with the participation of EG.
There have been works on the conductivity of different ions in EG aqueous solutions. For example, the transport properties of zinc sulfate and symmetrical 1 : 1 and 2 : 2 electrolytes in an EG–water binary system (EGW) were studied in [17]. The experimental data in these works were analyzed using the Lee–Waton equation, and the values of limiting molar conductivity, association constants, and the distances between the centers of ion pairs were obtained. The Stokes radius was calculated from the limiting ionic conductivities. Results were obtained for the model system (EG + H2O + CO2 + Na2CO3 + NaHCO3), which is important for understanding and simulating similar systems [18], but there are data for carbonates and hydrogen carbonates only.
It should be noted that there are either no data on directly measuring the electrical conductivity of EGW systems, or they are unsystematic. For example, there are data on measuring the concentration dependence of specific electric conductivity for an EGW mixture in the 0.3 to 1 range of concentrations [19]. This dependence shows there is a monotonic reduction in electrical conductivity when the amount of EG is raised. As is shown below, however, this dependence has a pronounced maximum.
The authors of [20] determined the activation energies of the electrical conductivity of a EGW binary mixture at three concentrations, which obviously does not allow us to draw any conclusions as to the effect composition has on activation energies.
Nevertheless, analyzing the concentration dependences of electrical conductivity and viscosity along with their activation energies allows us to study structure, solvation processes, and mechanisms of proton transfer to determine the effect the structure of an EGW chain has on proton conductivity at different concentrations. Although a theory of proton conductivity in aqueous solutions was proposed more than two hundred years ago [21], the mechanisms of proton transfer have been understood and explained only in the last two decades.
The EGW system is one of the simplest that can simultaneously be considered a model and a system for the production of new materials with conductivity comparable to (and even higher than) that of electrolytes, while being much less chemically aggressive.
This work is a first part of an analysis of the temperature and concentration dependences of the viscosity and electrical conductivity of an EGW binary system. We comparatively analyze the concentration dependences of viscosity and conductivity at 25°C to determine the change in structure for the complexes formed in the binary mixture upon a change in its composition.
EXPERIMENTAL
EGW mixtures of various concentrations were prepared by weighing on electronic scales (accuracy of measurement, ±0.0001 g). Bidistilled water was used to prepare the solutions. The purity of water was monitored by measuring its electrical conductivity, which was no more than 1.0 μS/cm at 20°C. The error in determining the molar content of each liquid was no more than ±0.0001. Chemically pure ethylene glycol was provided by ZAO EKOS-1.
The viscosities of pure liquids and binary mixtures were measured on a SV-10 vibrational viscometer (AND, Japan). The samples were placed into a thermostatic bath. The accuracy of temperature measurements was ±0.02 K. The error in measuring the viscosity coefficient was no more than 3%.
The specific electrical conductivity of the investigated solutions and pure liquids was determined on an HI 2300 submerged four-contact conductometer (Hanna Instruments, United States) equipped with an HI 76310 probe.
RESULTS AND DISCUSSION
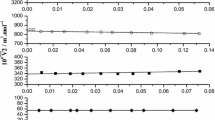
Figure 1 and Tables 1 and 2 show the experimental concentration dependences of viscosity \(\eta \) and specific electric conductivity \(\kappa \) for an EGW mixture at 25°C.
There are several ways to interpret the concentration dependences of viscosity that require the introduction of some adjustable parameters [22]. One of the most common ways is to use excessive viscosity \({{\eta }^{E}}\), which is the difference between the viscosity of a mixture and its components:
where \({{\eta }_{{\text{W}}}}\) and \({{\eta }_{{{\text{EG}}}}}\) are the viscosities of pure water and EG, respectively, and \(x\) is the mole fraction of EG. Figure 2 shows excess viscosity \({{\eta }^{E}}\) as a function of the amount of EG in a mixture.
Dependence of excessive viscosity \({{\eta }^{E}}\) and excess electrical conductivity \({{\kappa }^{E}}\) for EGW mixtures. The graph shows the concentration values corresponding to \({{\eta }^{E}}\) and \({{\kappa }^{E}}\) extreme values: the maximum excess conductivity at \(x = 0.11\) and minimum excess viscosity at \(x = 0.46\). (For for clarity, only solid lines are shown.)
We can also introduce excess electrical conductivity for a comparative analysis of viscosity and conductivity:
where \(\kappa \), \({{\kappa }_{{\text{W}}}}\), and \({{\kappa }_{{{\text{EG}}}}}\) are the specific electrical conductivities for a mixture and its pure components. Figure 2 also shows the concentration dependence of excess electrical conductivity.
It should be noted that excess electrical conductivity is not a universal parameter, so it is introduced for general consideration of different characteristics in a single scale. The electrical conductivities for pure components are in this case much weaker and have no appreciable effect on the concentration dependence of electrical conductivity.
There are two critical concentrations in the system (Fig. 2) that correspond to maximum excess electrical conductivity \({{\kappa }^{E}}\) and minimum excess viscosity \({{\eta }^{E}}\) when the concentrations are \({{X}_{1}} = 0.11\) and \({{X}_{2}} = 0.46\), respectively.
Grunberg and Nissan described the dynamic viscosity for binary mixtures as a function of their pure components [23]. They proposed a simple equation containing one adjustable parameter that was an attribute of intermolecular interaction between the components. Equation (1) allows determination of Grunberg–Nissan parameter \(G\), which is considered a measure of the intensity of interaction between different molecules:
Using Eq. (1), parameter \(G\) was found for each \(x\) of the mixture:
Figure 3 shows the relationship between parameter \(G\) and the composition of the mixture at 25°C. The data shows there are three regions, two in which parameter \(G\) varies almost linearly but with different slopes of the approximation lines, and one where parameter \(G\) has an extremum. The concentrations separating these regions are much closer to those given in Fig. 2 for excess viscosity and electrical conductivity values; i.e., \({{X}_{1}} = 0.11\) and \({{X}_{2}} = 0.46\). These concentrations correspond to those obtained in [24], where molecular dynamics was used to analyze the structure and characteristics of a hydrogen bond network for an EGW mixture at concentrations of 0 to 1 with a step of 0.1.
The concentration dependence of parameter \(G\) thus allows us to create an analytical description; this problem nevertheless remains beyond the scope of this work, since the behavior of parameter \(G\) when \(x < 0.1\) requires additional study. The behavior of \(G\left( x \right)\) in this area depends strongly on the viscosity of pure water and varies considerably within the measuring error, making it impossible to draw any clear conclusions. It should be noted, however, that the presented results are in good agreement with those for the methanol–water system in [25].
The concentration dependence of viscosity for an ideal liquid mixture consisting of similar molecules is described by the equation [26]
which corresponds to Eq. (1) when \(G = 0\). However, the experimental viscosity for a mixture of liquids consisting of different molecules differs greatly from the law determined using Eq. (2). The formation of complexes in solution raises the viscosity of a mixture, compared to the theoretical value obtained with Eq. (2). Figure 4 compares the experimental data and viscosity of an ideal mixture.
Comparison between (1) the experimental concentration dependence for the viscosity of an EGW mixture and (2) the theoretical value calculated with Eq. (2).
The difference between viscosities is in fact due to the nonideality of the system caused by complexes forming between water and EG molecules. Experimental studies performed by various means that often include computer simulations confirm complexes form in EGW mixture [4, 5].
We may therefore conclude that concentrations \({{X}_{1}} = 0.11\) and \({{X}_{2}} = 0.46\) divide the ranges of concentration where the molecular complexes have different structures; i.e., varying Grunberg–Nissan parameter \(G\) corresponds to a change in the interaction between water and EG upon changing the composition of the mixture.
The effect concentration has on the interaction between molecules was determined quantitatively in [24], where it was shown that the average number of hydrogen bonds per alcohol molecule was very large when the concentration of EG was low: 4.79 OH bonds per EG molecule when \(x = 0.1\). We may therefore assume that chains (clusters) of 8–9 water molecules, to the ends of which EG molecules are attached, form when the concentration of EG is \({{X}_{1}} = 0.11\). With monohydric alcohols (methyl, ethyl, and propyl), these chains are closed and fairly stable complexes form, resulting in the maximum concentration dependence of viscosity. These chains are not closed in polyhydric alcohols, which allows exchange with other complexes. Since such a structure corresponds to the maximum electrical conductivity, we may assume that proton transfer in such structures can occur along several water molecules, i.e., over long distances without the need to rotate the molecules to create the favorable conditions required for proton transfer. EG molecules are in this case responsible for the transfer of protons between the complexes.
To support this model, we can cite the results from [24], according to which mixed (EG–H2O) bonds predominate over the similar (EG–EG) bonds for EG in this range of concentrations, and proton transfer in most cases proceeds through EG oxygen.
A further increase in the amount of EG results in the breakdown of water clusters and increases the self-association of alcohol. These structures are more stable and the excess viscosity thus begins to grow. When \(x > 0.46\), mixed bonds (H2O–EG) start to dominate for mixed water molecules. In such a structure, protons should be transferred between water and alcohol molecules in stages, and the rotation of the molecules determines the rate of proton transfer. Figure 2 shows that when \(x > 0.46\), the electrical conductivity of the mixture changes very slowly, approaching the value of electrical conductivity for pure EG.
CONCLUSIONS
We analyzed the concentration dependences of the viscosity and specific electrical conductivity of an ethylene glycol–water system in order to interpret intermolecular interactions and the formation of complexes through hydrogen bonds. To obtain a more complete picture, it is obviously necessary to analyze the energy characteristics that depend on composition of the system. The aim of our next study will therefore be to analyze the temperature dependences of viscosity and conductivity for the system, and thus their activation energies.
REFERENCES
L. Saiz, J. A. Padro, and E. Guardia, J. Chem. Phys. 114, 3187 (2001).
M. A. Krest’yaninov, A. G. Titova, and A. M. Zaichikov, Russ. J. Phys. Chem. A 88, 2114 (2014).
N. K. Balabaev, S. V. Kraevskii, M. N. Rodnikova, and I. A. Solonina, Russ. J. Phys. Chem. A 90, 1000 (2016).
A. Jindal and S. Vasudevan, J. Phys. Chem. B 121, 5595 (2017).
R. M. Kumar, P. Baskar, K. Balamurugan, et al., J. Phys. Chem. A 116, 4239 (2012).
S. S. N. Murthy, J. Phys. Chem. B 104, 6955 (2000).
M. N. Rodnikova, N. A. Chumaevskii, V. M. Troitskii, and D. B. Kayumova, Russ. J. Phys. Chem. A 80, 826 (2006).
W. Caminati and G. Corbelli, J. Mol. Spectrosc. 90, 572 (1981).
K. A. Petterson, R. S. Stein, M. D. Drake, et al., Magn. Reson. Chem. 43, 225 (2005).
W. S. Price, H. Ide, and Y. Arata, J. Phys. Chem. A 107, 4784 (2003).
S. G. Kandlikar and M. Bulut, ASME. J. Heat Transfer 125, 317 (2003).
Y.-H. Zhou, S.-N. Li, Q.-G. Zhai, et al., J. Chem. Thermodyn. 42, 764 (2010).
R. Masoudi, B. Tohidi, A. Danesh, et al., Chem. Eng. Sci. 60, 4213 (2005).
E. V. Butyrskaya, N. V. Belyakova, M. V. Rozhkova, and L. S. Nechaeva, Russ. J. Phys. Chem. A 86, 1721 (2012).
C. Yang, P. Ma, F. Jing, et al., J. Chem. Eng. Data 48, 836 (2003).
T. Sun and A. S. Teja, J. Chem. Eng. Data 48, 198 (2003).
N. G. Tsierkezos and I. E. Molinou, Z. Phys. Chem. 216, 961 (2006).
P. L. Fosbol, K. Thomsen, and E. H. Stenby, Ind. Eng. Chem. Res. 48, 4565 (2009).
B. Sesta and M. L. Berardelli, Electrochim. Acta 17, 915 (1972).
D. Nowak-Wozny and T. Maczka, J. Electr. Eng. 58, 55 (2007).
C. J. T. de Grotthuss, Ann. Chim. (Paris) 58, 54 (1806).
A. Pal and G. Dass, J. Chem. Eng. Data 45, 693 (2000).
L. Grunberg and A. H. Nissan, Nature (London, U.K.) 164, 799 (1949).
A. Kaiser, M. Ritter, R. Nazmutdinov, et al., J. Phys. Chem. B 120, 10515 (2016).
M. Guettari and A. Gharbi, Phys. Chem. Liq. 49, 459 (2011).
T. Erdey-Gruz, Transport Phenomena in Aqueous Solutions (Akademiai Kiado, Budapest, 1974; Mir, Moscow, 1976).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Tulyabaev
Rights and permissions
About this article
Cite this article
Loskutov, V.V., Kosova, G.N. Molecular Structure of an Ethylene Glycol–Water Solution at 298 K. Russ. J. Phys. Chem. 93, 260–264 (2019). https://doi.org/10.1134/S003602441902016X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602441902016X