Abstract
Polyol synthesis of thin silver nanowires has been studied and their suitability for the formation of transparent electrodes has been shown. The influence of stepwise heating of the reaction system on the position and shape of the absorption band associated with the surface plasmon resonance of the formed silver nanostructures has been determined. Using X-ray powder diffraction analysis, it has been found that the material does not contain crystalline impurities and has a face-centered cubic lattice. According to the scanning and transmission electron microscopy data, the main fraction is represented by elongated nanostructures with 10–15 μm length (however, there are also structures with length up to 20 μm) characteristic for silver nanowires of the arc-shaped type. It is shown that the Ag nanowires obtained are quite thin (diameter is about 35–45 nm). In addition, some amount of microrods of 1–3 µm length is observed in the composition of the material, the diameter of which grows from 70 to 150 nm with decreasing length. In smaller quantities there is also an admixture of zero-dimensional particles, which are polyhedrons of various complexity. Atomic force microscopy has been used to study the surface of the film based on the obtained silver nanowires and the diameter of individual nanowire has been estimated. The optical properties and surface resistivity of the films based on the obtained silver nanowires have been examined. It has been found that the increase in transmittance at 550 nm from 73.9 to 90.3% is accompanied by an increase in the resistance value from 25 to 146 Ω/sq.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The dynamic development of modern microelectronics devices [1, 2], photovoltaics [3, 4], alternative energy [5, 6], optics [7, 8], biosensors [9, 10], and medicine [11, 12] requires improvement of technologies for creating transparent electrodes capable of exhibiting high UV-visible transmittance, low sheet resistance and low roughness, thermal stability, and mechanical flexibility, which is important in the development of flexible and wearable devices. Currently, the most widely used transparent electrodes based on the In2O3–SnO2 system (indium tin oxide, ITO) have low surface resistance (~10 Ω/sq) and high transmittance (>80%) in the visible range [13]. However, the high cost of indium, as well as the limited resistance of ITO films to bending/unbending cycles, which is reflected in cracking and a decrease in the electrical conductivity of electrodes, reduce the prospects for their further effective use in the creation of commercially available flexible electronic devices [14]. As a worthy alternative to ITO-based electrodes, silver nanowires (AgNWs) are increasingly being considered; they are extended nanostructures with a high aspect ratio (up to several thousand [15]) and characterized by low resistance (including ~2.5 Ω/sq [14]), high optical transmittance (up to 91% [16]) and mechanical flexibility (~1000 bending/unbending cycles [17]). Various research papers devoted to the formation of transparent electrically conductive coatings based on silver nanowires report that the factors having a decisive influence on the optical and electrophysical properties of the final electrodes are the microstructure of AgNWs (aspect ratio, uniformity, and roughness), their chemical purity and stability, as well as uniform distribution of nanowires over the surface of the substrate during film deposition [18–22].
One of the most popular and promising methods for the synthesis of silver nanostructures with various morphologies, including ones in the form of one-dimensional nanoobjects, is the polyol method [23]. The advantages of this approach include the ability to control the morphology and kinetics of the nanowire formation process, achieving a high practical yield of the target product in a relatively short synthesis time (usually less than 1 h), as well as proven efficiency in terms of scaling and integration into industrial production [17 , 24]. It was previously demonstrated [25–27] that temperature is one of the key parameters of polyol synthesis that affects the microstructural features of its target products. This pattern is due to an increase in the reducing ability of the polyhydric alcohols used in this case with increasing temperature of the reaction system. It was shown [28] that when ethylene glycol is used as a solvent, the process of its conversion to glycol aldehyde, which is an active reducing agent, occurs at elevated temperatures (>150°C). Thus, in the temperature range of 130–150°C, the formation of silver nanorods is often observed, and a temperature of 170°C in some cases is considered optimal for the formation of nanowires with a high aspect ratio and significant practical yield. A further increase in temperature (up to 190 and 200°C), as a rule, negatively affects the formation of elongated Ag nanostructures, leading to a decrease in the aspect ratio and the appearance of a significant number of zero-dimensional particles. At a lower temperature (110°C), the formation of silver particles with a close to spherical shape is observed. The authors [29] observed a similar dependence and hypothesized that the relatively low processing temperature does not provide the system with sufficient energy to activate the specific facets required for the anisotropic growth of nanowires. Despite the active study of the influence of the temperature factor on the kinetics and mechanism of polyol synthesis of AgNWs, the optimal temperature ranges for the formation of silver nanowires with a high aspect ratio differ significantly among different teams of authors [28, 30–33], which indicates the relevance of additional research.
Thus, the aim of this work is to study the process of polyol synthesis of silver nanowires during stepwise heating of the reaction system, as well as to evaluate their suitability for the formation of electrodes with a high level of optical transparency in the visible range and low surface resistance.
EXPERIMENTAL
The synthesis of silver nanowires was carried out using the polyol method with stepwise heating of the reaction system. At the first stage, the following solutions were prepared: polyvinylpyrrolidone (0.090 g) in ethylene glycol (11 mL), silver nitrate (0.045 g) in ethylene glycol (1.5 mL), a solution of ferric chloride in ethylene glycol with a concentration of iron cations of 3 × 10–3 mol/L. Next, the polyvinylpyrrolidone solution was heated with stirring (the stirring intensity was 80 RPM) in an oil bath to 150°C and the ferric chloride solution (0.625 mL) was added to it as a source of chloride anions. Then, by adding the silver nitrate solution to the reaction system, the process of formation of primary silver nanoparticles was initiated; as a result, the solution acquired a yellow tint. Then the heat treatment of the system was continued for 30 min. After this, the temperature of the reaction system was increased to 160°C and maintained for 30 min. At the next stage, the system was heated in a similar way to 170°C and heat treatment was also carried out for 30 min.
During the polyol synthesis, samples of 0.5 mL were taken from the reaction system every 30 min and the solid phase was separated from the mother solution by centrifugation. After removing the mother liquor, the precipitate was dispersed in ethyl alcohol to purify it from polyvinylpyrrolidone, followed by separation of silver nanostructures. To homogenize the sediment in ethyl alcohol, the system was processed using a vortex. The procedure for purification of metal particles was repeated three times.
To evaluate the optical characteristics of the formed silver nanostructures, we used an SF-56 UV-Vis spectrophotometer (OKB Spectr) to record absorption spectra of samples diluted 50 times with distilled water taken every 30 min of heat treatment of the reaction system. The spectra were recorded in the wavelength range 300–700 nm with a resolution of 1 nm using a 10 mm thick quartz cell.
Films based on the resulting silver nanowires were deposited onto the surface of glass substrates 25 × 10 × 1 mm in size by the drop-casting method using an appropriate disperse system in ethyl alcohol (the mass fraction of AgNWs was ~0.2%), varying the sample volume in the range of 7–20 μL. The formed films were dried at room temperature (22 ± 2°C) for 10 min. The surface resistance of AgNWs-based films was measured using a laboratory multimeter and a specialized cell.
The crystal structure of the film based on the obtained silver nanowires was studied by X-ray powder diffraction in the 2θ angle range of 20°–80° on a Bruker D8 Advance diffractometer (CuKα radiation, λ = 1.5418 Å, a Ni filter, E = 40 keV, I = 40 mA) with a resolution of 0.02° and signal accumulation time at a point of 0.3 s.
The features of the microstructure of the resulting nanowires were studied using scanning electron microscopy (SEM; dual-beam scanning electron-ion microscope FIB-SEM Tescan Amber, accelerating voltage 2 kV) and transmission electron microscopy (TEM; Jeol Jem-1011 with an Orius Sc1000w digital camera).
Atomic force microscopy (AFM) of the resulting silver nanostructures deposited on an aluminum substrate by the drop-casting method was carried out on a Solver-Pro microscope (NT-MDT, Zelenograd, Russia). All measurements were performed in air in semi-contact mode using an ETALON HA_HR series probe with a tip rounding radius of <35 nm and a resonant frequency of 210 kHz.
RESULTS AND DISCUSSION
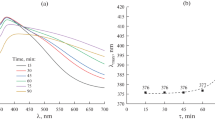
Due to the fact that silver nanostructures are characterized by surface plasmon resonance (SPR), studying the relationship between their microstructural and optical properties is an important task in materials science. Therefore, to monitor the formation of the corresponding nanoparticles and their microstructural evolution, it is often convenient to use UV-Vis spectrophotometry. As can be seen from the absorption spectra of samples taken during polyol synthesis (Fig. 1a), stepwise heating of the reaction system accompanied by holding for 30 min at the appropriate temperature leads to noticeable changes in the position and shape of the absorption band associated with the manifestation of SPR. Thus, for a sample obtained as a result of heat treatment at 150°C for 30 min, an absorption band with a maximum at 410 nm is observed associated with the surface plasmon resonance of primary silver nanoparticles [34]. Further heating of the system and its heat treatment for 30 min at 160°C lead to significant changes in the shape of the absorption band, as well as an increase in its intensity. In particular, the maximum of the absorption band shifts to a shorter wavelength region of the spectrum (up to 377 nm) and a shoulder appears around 354 nm, which is characteristic of fairly thin AgNWs and can be attributed to the transverse SPR modes of nanowires with a pentagonal cross section. Thus, it can be assumed that at this stage the active formation of silver nanowires begins. Nevertheless, a signal is also observed in the spectrum at a wavelength of >400 nm, indicating the preservation of a significant amount of primary silver nanoparticles, which indicates the incompleteness of the process of formation of silver nanowires. Increasing the temperature to 170°C and additional heat treatment lead to a minimization of the contribution from primary silver nanoparticles, a slight increase in the intensity of the main absorption band, and an even more significant increase in the intensity of the shoulder around 354 nm. The shape of the absorption band of the final reaction system suggests that the main process of formation of AgNWs has been completed, and an additional shift of the maximum to the short-wavelength region of the spectrum (up to 376 nm) indicates that rather thin nanowires with a diameter of <50 nm were formed during the polyol synthesis [35].
The crystal structure of a film based on synthesized AgNWs deposited on the surface of a glass substrate (sample volume 20 μm) was studied using X-ray powder diffraction (Fig. 1b). As a result, two reflections were recorded for the formed film related to the crystallographic planes (111) and (200) of the face-centered cubic silver lattice (space group Fm\(\bar {3}\)m, JCPDS #99-0094). It should be noted that a wider range of reflections is most common in the literature for these materials. In this regard, the silver nanowires we obtained appear to be characterized by some specific crystal structures, when crystal growth along the (220), (311), and (222) planes is limited. These results indirectly indicate texturing of the material and the formation of anisotropic nanostructures. This is also indicated by the increased value of the ratio of signal intensities (111) and (200), which amounted to 4.7 (compared to the theoretical value of 2.5). However, X-ray powder diffraction results are not sufficient confirmation of the formation of particles with a certain microstructure, and additional methods should be used. Generally, silver nanowires undergo partial oxidation during synthesis, storage and use, which can lead to a deterioration in their electrical conductivity. In our case, as can be seen from the X-ray powder diffraction pattern, the silver oxide impurity is not detected, and there are signals only from metal nanostructures.
The microstructure of silver nanowires obtained by the polyol method was studied by SEM. The micrographs obtained (Fig. 2a) indicate the formation of material with the target morphology. The main fraction consists of extended nanostructures 10–15 μm long with an arched shape characteristic of AgNWs, which is in good agreement with the X-ray powder diffraction results. In addition to silver nanowires, nanorods 1–3 μm long are observed, which have an increased diameter compared to nanowires (~80 nm), as well as a small number of zero-dimensional particles. According to the results obtained (Fig. 2b), in some cases, silver nanowires are connected by side faces, which, during heat treatment of the reaction system, can probably lead to their recrystallization and the formation of one-dimensional structures of larger diameter. This combination of AgNWs can be accompanied by the appearance of defects in the crystal structure and, consequently, the appearance of microstresses, resulting in the destruction of a large nanowire. Therefore, apparently, impurities are formed, which are microrods and zero-dimensional particles 100–200 nm in size. At higher magnification, it is clear that the resulting silver nanowires are very thin (with a diameter of ~35–45 nm), which is in good agreement with the results of UV-Vis spectrophotometry (the position of the maximum of the main absorption band at 376 nm indicates the formation of thin nanowires). It should be noted that the literature often reports the need to complicate the composition of the reaction system to form silver nanowires with a diameter of <50 nm, which are preferable for obtaining conductive films characterized by high electrical conductivity and low absorption in the visible range of the electromagnetic spectrum. In our case, the formation of thin AgNWs can be explained by the peculiarities of the heat treatment regime. Analysis of the surface of the formed one-dimensional nanostructures indicates that they have faceted side surfaces; this is most noticeable for nanorods with a larger diameter. Thus, the features of the microstructure of the material additionally indicate a well-formed crystal structure.
For a more detailed analysis of the microstructure of the resulting material, TEM was used (Fig. 3). The results obtained are in good agreement with SEM data, in particular, the resulting silver nanowires have an average length of ~10–15 μm, but longer structures are also found (up to 20 μm long ). The material also contains a number of microrods 1–3 μm long, the diameter of which increases from 70 to 150 nm as the length decreases (Figs. 3b, 3c). An admixture of zero-dimensional particles, which are polyhedra of varying complexity, is found in smaller quantities. These impurity structures (microrods and zero-dimensional particles) can be separated from the main part of silver nanowires by fractionation in order to achieve higher transparency of the corresponding conductive films. The TEM results made it possible to determine more accurately the diameter of the formed AgNWs (Fig. 3d). It can be seen that the majority of one-dimensional nanostructures are characterized by a diameter of 35–45 nm; this is confirmed by SEM and UV-Vis spectrophotometry data. In addition, the TEM results clearly indicate that the AgNWs have faceted side surfaces, as was observed when studying their microstructure using the SEM method.
The surface of the film based on the resulting silver nanowires was studied using AFM. The corresponding topographic images (Figs. 4a–4c) confirm the nature of the microstructure of the material observed using electron microscopy. The image of the surface area of 225 μm2 shows (Fig. 4a) that the film is a network of 3–5 layers of mutually intersecting one-dimensional nanostructures ~10 μm long, and the maximum height difference over the indicated area is ~450 nm, which is comparable to the value of this parameter for a pure substrate. Thus, the film under study is quite thin and homogeneous. As shown before, in addition to Ag nanowires, the material contains a certain amount of microrods, as well as zero-dimensional particles. Due to the capabilities of the method, which consist in three-dimensional scanning of the surface under study, the microstructural features of an individual nanowire were studied (Fig. 4c) and a profile along the cross section was constructed (Fig. 4d). It can be seen that in this case there is an overestimated value of the diameter (~200 nm) due to the geometric characteristics of the probe used. The results of measurements along axis z in this case are more correct; they made it possible to estimate the diameter of an individual silver nanowire at a level of ~35 nm, which corresponds to the value of this parameter determined by SEM and TEM methods. It also follows from the AFM data that, due to the sufficiently long length of the AgNWs, relatively small defects in the substrate relief can be compensated and will not have a significant negative effect on the electrical properties of the film formed.
The optical properties of films based on the resulting silver nanowires deposited by the drop-casting method on the surface of glass substrates were studied using UV-Vis spectrophotometry (Fig. 5a). For the glass substrates used, as the radiation wavelength decreases <350 nm, the absorption increases significantly, which is typical for these materials. In this case, the transmittance at 550 nm for a pure substrate is 91.3%. As can be seen from the spectra, for all films based on AgNWs, a characteristic absorption band is observed, which is related to the plasmon resonance of the corresponding one-dimensional nanostructures. As the volume of the disperse system used to form the film increases, the transmission decreases over the entire wavelength range under study. It should be noted that the maximum of the main absorption band with an increase in the used volume of dispersion noticeably shifts towards longer wavelengths, from 352 (for a dispersion volume of 7 μL) to 358 nm (20 μL). This also leads to a gradual decrease in transmittance at 550 nm: 90.3 (7 μL), 88.2 (10 μL), 81.3 (15 μL), and 73.9% (20 μL). For films based on silver nanowires obtained in this way, the surface resistance (Rs) was assessed. As can be seen from Fig. 5b, an increase in transmittance at 550 nm (T550 nm) is accompanied by an increase in the value of Rs according to a logarithmic law. Thus, at T550 nm = 73.9% (the volume of the disperse system is 20 μL), the surface resistance value is 25 Ω/sq. As the transmittance increases to 81.3%, the Rs value also increases, but remains at a relative low level (33 Ω/sq). For a film characterized by a transmittance of 88.2%, the surface resistance increases to 56 Ω/sq, and at T550 nm = 90.3% this parameter is 146 Ω/sq. The indicated results on the relationship between transparency and electrical conductivity of films based on the obtained silver nanowires are in good agreement with the literature data and indicate that these characteristics meet the requirements for transparent conductive films [36].
Thus, as a result of the study, the process of polyol synthesis of thin silver nanowires with stepwise heating of the reaction system was studied, and their suitability for the formation of transparent electrodes with high transparency in the visible range and low surface resistance was shown. To increase the transparency of the resulting films, the silver nanowires used can be additionally purified from microrods and zero-dimensional particles present in a certain amount by fractionation.
CONCLUSIONS
Here, we have studied the process of synthesizing thin silver nanowires using the polyol method and demonstrated their suitability for the formation of transparent electrodes. It has been shown that stepwise heating of the reaction system (150 → 160 → 170°C) leads to noticeable changes in the position and shape of the absorption band associated with the manifestation of surface plasmon resonance of the resulting silver nanostructures. Using X-ray diffraction, only two reflections have been detected for the resulting silver nanowires related to the crystallographic planes (111) and (200) of the face-centered cubic lattice of silver. At the same time, no crystalline impurities, including silver oxide, have been found in the composition of the material. According to SEM data, the main fraction consists of extended nanostructures 10–15 μm long with an arched shape characteristic of AgNWs. In some cases, silver nanowires are connected by side faces, which, during heat treatment of the reaction system, can probably lead to their recrystallization and the formation of one-dimensional structures of larger diameter. It has been found that the resulting AgNWs are very thin (diameter ~35–45 nm), which is in good agreement with the results of UV-Vis spectrophotometry. TEM results also indicate that the resulting silver nanowires have an average length of ~10–15 μm, but longer structures (up to 20 μm in length) are also observed. The material also contains a number of microrods 1–3 μm long, the diameter of which increases from 70 to 150 nm as the length decreases. An admixture of zero-dimensional particles, which are polyhedra of varying complexity, is found in smaller quantities. Using AFM, the surface of a film based on the resulting silver nanowires has been studied and the diameter of an individual silver nanowire has been estimated to be ~35 nm. It also follows from the AFM results that due to the sufficiently long length of the AgNWs, relatively small defects in the substrate topography can be compensated and will not have a significant negative effect on the electrical properties of the formed film. As part of the study, the optical properties and surface resistance of films based on the resulting silver nanowires have been studied. It has been shown that an increase in transmittance at 550 nm from 73.9 to 90.3% is accompanied by an increase in Rs from 25 to 146 Ω/sq. The results obtained on the relationship between transparency and electrical conductivity of the films are in good agreement with the literature and indicate that these characteristics meet the requirements for transparent conductive films.
REFERENCES
D. Shukla, Y. Liu, and Y. Zhu, Nanoscale 15, 2767 (2023). https://doi.org/10.1039/D2NR05840E
L. Zhang, T. Song, L. Shi, et al., J. Nanostructure Chem. 11, 323 (2021). https://doi.org/10.1007/s40097-021-00436-3
J. Yang, F. Yu, A. Chen, et al., Adv. Powder Mater. 1, 100045 (2022). https://doi.org/10.1016/j.apmate.2022.100045
J.-J. Shen, Synth. Met. 271, 116582 (2021). https://doi.org/10.1016/j.synthmet.2020.116582
W. Zhao, M. Jiang, W. Wang, et al., Adv. Funct. Mater. 31, 2009136 (2021). https://doi.org/10.1002/adfm.202009136
S. Kiruthika, N. Sneha, and R. Gupta, J. Mater. Chem. A 11, 4907 (2023). https://doi.org/10.1039/D2TA07836H
L. Huang, X. Chen, X. Wu, et al., Flex. Print. Electron. 8, 025021 (2023). https://doi.org/10.1088/2058-8585/acdb84
J. Lee, Y. Lee, J. Ahn, et al., J. Mater. Chem. C 5, 12800 (2017). https://doi.org/10.1039/C7TC04840H
Y. Wang, J. Kong, R. Xue, et al., Nano Res. 16, 1558 (2023). https://doi.org/10.1007/s12274-022-4757-9
D. E. Oh, C.-S. Lee, T. W. Kim, et al., Biosensors 13, 704 (2023). https://doi.org/10.3390/bios13070704
J. Jang, J. Kim, H. Shin, et al., Sci. Adv. 7 (2021). https://doi.org/10.1126/sciadv.abf7194
V. H. Nguyen, D. T. Papanastasiou, J. Resende, et al., Small 18, 2106006 (2022). https://doi.org/10.1002/smll.202106006
A. Elsokary, M. Soliman, F. Abulfotuh, et al., Sci. Rep. 14, 3045 (2024). https://doi.org/10.1038/s41598-024-53286-8
D. Kumar, V. Stoichkov, E. Brousseau, et al., Nanoscale 11, 5760 (2019). https://doi.org/10.1039/C8NR07974A
Z. Fan, J. Wang, L. He, et al., Langmuir 39, 10651 (2023). https://doi.org/10.1021/acs.langmuir.3c01264
C. Preston, Z. Fang, J. Murray, et al., J. Mater. Chem. 2, 1248 (2014). https://doi.org/10.1039/C3TC31726A
Y. Zhu, Y. Deng, P. Yi, et al., Adv. Mater. Technol. 4, 1900413 (2019). https://doi.org/10.1002/admt.201900413
Q. Liao, W. Hou, J. Zhang, et al., Coatings 12, 1756 (2022). https://doi.org/10.3390/coatings12111756
L. Shi, Micro Nano Lett. 18, e12151 (2023). https://doi.org/10.1049/mna2.12151
S. Hemmati, M. T. Harris, and D. P. Barkey, J. Nanomater. 2020, 1 (2020). https://doi.org/10.1155/2020/9341983
X. Duan, Y. Ding, and R. Liu, Mater. Today Energy 37, 101409 (2023). https://doi.org/10.1016/j.mtener.2023.101409
Y. H. Wang, X. Yang, D. X. Du, et al., J. Mater. Sci. Mater. Electron. 30, 13238 (2019). https://doi.org/10.1007/s10854-019-01687-1
S. Fahad, H. Yu, L. Wang, et al., J. Mater. Sci. 54, 997 (2019). https://doi.org/10.1007/s10853-018-2994-9
F. Fiévet, S. Ammar-Merah, R. Brayner, et al., Chem. Soc. Rev. 47, 5187 (2018). https://doi.org/10.1039/C7CS00777A
P. Zhang, I. Wyman, J. Hu, et al., Mater. Sci. Eng. B 223, 1 (2017). https://doi.org/10.1016/j.mseb.2017.05.002
T. Araki, J. Jiu, M. Nogi, et al., Nano Res. 7, 236 (2014). https://doi.org/10.1007/s12274-013-0391-x
S. M. Bergin, Y.-H. Chen, A. R. Rathmell, et al., Nanoscale 4, 1996 (2012). https://doi.org/10.1039/c2nr30126a
S. Coskun, B. Aksoy, and H. E. Unalan, Cryst. Growth Des. 11, 4963 (2011). https://doi.org/10.1021/cg200874g
Y. Sun, B. Gates, B. Mayers, et al., Nano Lett. 2, 165 (2002). https://doi.org/10.1021/nl010093y
F. Basarir, S. De, H. Daghigh Shirazi, et al., Nanoscale Adv. 4, 4410 (2022). https://doi.org/10.1039/D2NA00560C
Z. Kaili, D. Yongguo, and C. Shimin, J. Nanosci. Nanotechnol. 16, 480 (2016). https://doi.org/10.1166/jnn.2016.12158
X. Lai, X. Feng, M. Zhang, et al., J. Nanoparticle Res. 16, 2272 (2014). https://doi.org/10.1007/s11051-014-2272-y
J. Jiu, T. Araki, J. Wang, et al., J. Mater. Chem. A 2, 6326 (2014). https://doi.org/10.1039/C4TA00502C
H. Mao, J. Feng, X. Ma, et al., J. Nanoparticle Res. 14, 887 (2012). https://doi.org/10.1007/s11051-012-0887-4
E.-J. Lee, M.-H. Chang, Y.-S. Kim, et al., APL Mater. 1, 042118 (2013). https://doi.org/10.1063/1.4826154
H. Ha, C. Amicucci, P. Matteini, et al., Colloid Interface Sci. Commun. 50, 100663 (2022). https://doi.org/10.1016/j.colcom.2022.100663
Funding
The study was supported by the Russian Science Foundation, grant no. 23-79-10081, https://rscf.ru/en/project/23‑79‑10081/
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simonenko, N.P., Simonenko, T.L., Gorobtsov, P.Y. et al. Polyol Synthesis of Silver Nanowires and Their Application for Transparent Electrode Fabrication. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023624601697
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023624601697