Abstract
As a powerful strategy to precisely fabricate multifunctional materials with unique optoelectronic properties, donor-acceptor (D–A) alignment, which integrates D–A pairs into the modular and versatile crystalline cation metal–organic frameworks (CMOFs) and cation coordination polymers (CCPs) is applied at the molecular level. In this paper, a photo- and thermochromic CCP, named [Cd (PTA)0.5(bcbpy)0.5Br] (1), was successfully produced by the self-assembly of a photo- and thermoactive 1,1'-bis((3-carboxylatobenzyl)-4,4'-bipyridinium)-dibromo ligand (H2bcbpy·2Br) with cadmium chloride and terephthalic acid (PTA). This complex is characterized by single-crystal X-ray diffraction, powder X-ray diffraction and it exhibits high sensitivity to both heat and different illuminant. The exceptional difunctional characteristics of electron-transfer (ET) thermochromism and photochromism in 1 are mostly influenced by the photosensitivity and thermal sensitivity of H2bcbpy·2Br. Otherwise, during the coloration-decoloration phase, there is a photoluminescence “on/off switch” which would direct the synthesis of new crystals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Cation coordination polymers (CCPs) and cation metal-organic frameworks (CMOFs), which exhibit color-changing, are the most primary and available materials. Because their demands are increased in luminescence, chromic materials, proton conduction, and sensors, which have attracted tremendous attention [1–7]. Three-dimensional multichromic CCPs and CMOFs are an interesting class of porous sensing materials established from metal center coordinated with functional organic linkers. They have been synthesized by more and more researchers owing to their novel, outstanding compositional structural and functional diversity. Chromic materials have a good response to heat, light, X-rays, electricity, as well as other stimulate, which get wide attention, and have high application potential in organic photovoltaic, thermoelectric generators or light-emitting diodes [8–11]. Color-changing organic-inorganic hybrid materials are a kind of ground-breaking materials and have attracted much attention due to their possible application prospects [12]. Various chromic CCPs and CMOFs named after the stimulate, for example, thermochromic, photochromic, electrochromic, hydrochromism, etc. Furthermore, an interesting feature of this class of chromic materials is the discoloration step, which is the transfer of electrons from the electron donor (d) to electron acceptor (ET) resulted in the generation of free radicals [13–17]. In the development of chromic organic−inorganic hybrids materials, the optical properties of organic ligands are an important strategy for designing materials with multichromic and mumtifunctional applications [14, 15]. Many families of organic and inorganic multichromic materials have been discovered. Among them, the selection of organic species, which objects as the major development for the function of the materials [18–20].
Several chromic organic ligands (piperazine, divinene, naphthalenediimide and violone) have been studied in response to diverse external stimuli [10, 11]. Viologens/bipyridine derivatives (1,1'-disubstituted 4,4'-bipyridine) are widely used as herbicides in a different kind of drugs and medicines, have a long history of research and development, and are famous for their unique electron-lacking properties: one is to form charge transfer complexes with electron-rich substances (such as organic amines), and the other is to implement reversible electron transfer when were exposed to different kinds of stimuli. The electron-deficient viologen cations (V2+) can form vioiogen radicals (V˙) by single electron transfer from the suitable electron donor and are always with recognizable colour changing. To construct chromic viologen-based CCPs with the electron transfer (ET) progress, some key factors should be considered, such as the packing type, the distance and orientation between D and A, the hydrogen bonds and so on. But the design and choice of the viologen is the most important.
In this paper, the novel multichromic cation coordination polymer [Cd(BTC)0.5(bcbpy)0.5Br] (1) was synthesized from 1,1'-bis (3-cyanobenzene)-[4,4'-bipyridine]-1,1'-dibromide, CdBr2·4H2O and H4PTA as raw materials. Compound 1 has great photosensitive activity and is responsive to Uv light and blue light at room temperature. Compound 1 changes color under 365 nm UV light and 405 nm blue light. At the same time, compound 1 is thermochromic when heated at 90°C. The detection and recognition ability of aniline was demonstrated. Uv/vis diffuse reflectance spectra, electron spin resonance (ESR) and theoretucal study were used to increase available and viewpoints about the mechanisms of color rendering progression.
EXPERIMENTAL
All materials were bough commercially and there is no further purification. The viologen synthesis and instrument testing are in the supporting information. The synthetic route and 1H NMR spectrum of H2bcbpy·2Br (600 MHz, D2O) are in Figs. S1 and S2.
Synthesis of [Cd(PTA)0.5(bcbpy)0.5Br] (1). With CdBr2·4H2O (29 mg, 0.1 mmol), pyromellitic acid (25.4 mg, 0.1 mmol), H2bcbpy·2Br (54 mg, 0.1 mmol), DMF (2 mL) and distilled water (3 mL) was thermoscopically reacted to get the light white monocrystalline acicular framework of complex 1. The mixture was sealed in a 25 mL polytetrafluoroethylene reactor and heated at 90°C for 96 h. Next, the autoclave cooled slowly to indoor temperature. The crystals are gathered via filtration, cleaned with deionized water, subsequently, dried in air. Based on CdBr2·4H2O, the yield of violet ligand was 62% of C18H11O4N2BrCd. FT‑IR (KBr, cm–1): 3300–3500 m, 2260–2215sh, 1642vs, 1586vs, 1541vs, 1481vs, 1367vs, 1250–1140s, 816s, 578s. TGA curve (Fig. S3) shows that in the temperature before ca. 330°C, 1 exhibits a weight loss, this illustrated thatthere were no water and solvent DMF molecules in the framework, which was exactly the structure shown in Fig. 1 and the framework collapses at temperatures from ca. 330 to 700°C.
RESULTS AND DISCUSSION
Crystal Structure of [Cd (PTA)0.5(bcbpy)0.5Br] (1)
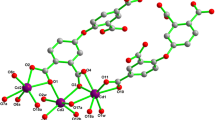
Single-crystal X-ray diffraction (XRD) analysis shows compound 1 crystallized in the triclinic system and its spatial group is P-1. Although this class crystals based on the host-guest cluster interaction has been widely reported, this 1D coordination frameworks with the ligand H2bcbpy·2Br extremely rare. The light-yellow crystal of 1 was obtained by unique design by the counter Br– viologen ligand and CdBr2·4H2O, which achieved the coordination of Br–(Fig. 1). In Fig. 1a, the structure of coordination environment of 1, the metal Cd coordinated with PTA4− ligand and formed 1D structure. The asymmetric unit was shown in Fig. S4, 1 have anionic framework [Cd (PTA)0.5Br]− half of the bcbpy2+cation resides in the channel, making up most of crystal 1 coordinated with five O atoms from PTA4− and one Br atom. Each PTA4− ligand links with six Cd atoms, as illustrated in Fig. 1b, Cd(1) and Cd(#1), Cd(4) and Cd(#4) are bidentate coordination with six O atoms from four carboxyl of PTA4−, Cd(2) and Cd(3) are monodentate coordination with one O atom from different carboxyl of PTA4−. Meanwhile, we found that the distance between O and N is 3.749 and 3.922 Å, and the distance from Br to the bipyridine ring of bcbpy2– viologen ligand is 3.529 Å displayed in Fig. 1c, they are likely to be pathways of electron transfer and arising the color change of 1 because of the viologen free radical generating [13, 14, 17]. The structure of 1D packing was displayed in Fig. 1d. Crystal data and framework refinements are given in Tables S1, S2 and S3.
Photo- and Thermochromic Behavior
Based on the response of H2bcbpy·2Br to UV light and blue light, the solid-state UV/visible diffuse reflectivity spectra of H2bcbpy·2Br ligands (Fig. S5) and crystal samples (Fig. 2) were measured and transformed into absorption spectrum to explore their chromic properties [21–23]. The absorption spectrum of hybrid material 1 shows a strong absorption band in the range of 250 ~ 800 nm, and the absorption of UV light is more potent than that of blue light under the same situation. As shown in Fig. 3, The light-yellow complex sample turns blue since irradiation upon UV light and blue ray. From these results, 1 has better response to UV light compared with the blue light, the decolorized complex could be decolorized after a week in the dark, and the decolorized complex can turn blue again after irradiation, which means that the photochromic behavior of the complex is reversible. The powder XRD profiles and infrared (IR) spectra show no appreciable variations after photochromism (Figs. S6 and S7), suggesting that the crystalline and molecular structures are maintained without bond cleavage or formation, which indicates that the origins of photo- and thermochromic properties for 1 are not photoinduced isomerization or photolysis. To further study the photochrmic mechanism of 1, electron paramagneticresonance (EPR) spectroscopy and theory study with Guassian 09 were implemented to ensure the involvement of viologen radicals in the chromic progress. As displayed in Fig. S8, the EPR spectroscopy was performed before and after irradiation by UV and blue light, which can explain the production of viologen radicals. Meanwhile, the estimation consequences of molecular frontier orbitals (HOMO and LUMO) also theoretically prove the generation of viologen radicals, which indicated O2– atoms from PTA4– and Br– as the donor and the N atom as the acceptor of electron (Fig. S9). Compound 1 changes from yellow to green when heated at 90°C for 2 min (Fig. S10), the EPR result exhibited that the production of viologen radicals, which arises the color changing of 1. From the structure of 1 (Fig. 1a), there was no free and coordination water molecule, so the color changing arises by heat.
Photoswitchable Luminescence Properties
Considering the luminescence performance of the H2bcbpy·2Br ligand, the photo-controlled luminescence properties are illustrated in our research as another product of the electron transfer step [18, 19]. Due to the better response to UV light. Compound 1 exhibits evident fluorescence quenching phenomenons under 365 nm UV light irradiation. As displayed in Fig. 4, the fluorescence spectra one demonstrates that when excited at 380 nm, there is a strong emission peak at 500 nm, which may be caused by D–A interactions between the electron donor and bcbpy ligand. In addition, under Uv irradiation, the emission peak decreases rapidly and gradually (365 nm, 40 W). Therefore, the emission intensity is influenced by the ET progress, which results in quenching luminescence. As we see, in Fig. 4, the fluorescent spectrum of hybrid 1 displays a broad, intense absorption band from 410 to about 700 nm, which is like the absorption band of UV/vis diffuse-reflectance spectrum in Fig. 2a. The consequences show that photochromism and photo-controlled luminescence could take place at the same time, which is caused by photoinduced electron transfer between the donor (O2– and Br–) and the acceptor (N+atom from pyridine of H2bcbpy·2Br ligand).
Sensing of Organic Ammonia
As we know, viologen ligands (H2bcbpy·2Br) could be regarded as a kind of Lewis acid (N+ cations in the pyridine ring). Meanwhile, H2bcbpy·2Br has good electron-accepting capabilities because of their electron-deficient properties. Meanwhile, ammonia is an excellent electron donor and Lewis’s base. Electron rich organic amine molecules can provide electronic properties to CCPs with the viologen moieties within the framework, resulting in a color change. Along these lines, the treatment between 1 and different organic ammonia like, primary ammonia (NH3, ethylamine and N-propylamine and secondary ammonia (diethyamine and dipropylamine) was done. In Fig. 5, when 1 exposures to five different organic ammonia vapors, like most of MOFs and CPs based viologen ligand, exhibits selective adsorption to different organic ammonia. When 1@NH3, ethylamine and N-propylamine, became light green, dark green and brown separately. However, to secondary ammonia, 1@diethyamine and dipropylamine amlost have no color change. From Fig. 1d, as we know, the N+ cation can form interaction with organic ammonia molecules. As follows from the PXRD recorded in situ, the solid saturated with crystalline 1@ammonia and the positions of lines are very close to the ones of the initial phase 1, the crystal structure is scarcely not destroyed (Fig. S11). The results illustrated that 1 has better adsorption for primary ammonia molecules based on the basic complex structure and occurs electrostatic adsorption with amine molecules, which have little size and volume, due to the color-changing of 1 in solid state.
CONCLUSIONS
All in all, in this article, a novel one-dimensional CCP composed of [Cd(PTA)0.5Br]− and bcbpy2+ guest cations has been prepared, named [Cd(PTA)0.5(bcbpy)0.5Br] (1). Compound 1 is photoactive and can react with 365 nm UV light and 405 nm blue light rapidly. The photochromic process of 1 formed from the generation of viologen radicals by photo-induced ET progress. Meanwhile, 1 exhibits thermochromism, photo-controlled luminescence and sensing of primary ammonia properties in the solid state.
REFERENCES
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 66, 1350 (2021). https://doi.org/10.1134/S0036023621090047
V. S. Gusarov, A. M. Cheplakova, D. G. Samsonenko, A. S. Vinogradov, and V. P. Fedin, Russ. J. Inorg. Chem. 66, 1374 (2021). https://doi.org/10.1134/S0036023621090035
Y. Y. Guo, R. D. Wang, W. M. Wei, F. Fang, L. Wang, S. S. Zhang, J. Zhang, L. Du, and Q. H. Zhao, Inorg. Chem. 63, 3870 (2024). https://doi.org/10.1021/acs.inorgchem.3c04228
M. Y. Guo, G. Li, S. L. Yang, R. Bu, X. Q. Piao, and E. Q. Gao, Chem. Eur. J. 27, 16415 (2021). https://doi.org/10.1002/chem.202102413
S. Chand, S. C. Pal, D. W. Lim, K. Otsubo, A. Pal, H. Kitagawa, and M. C. Das, ACS Mater. Lett. 2, 1343 (2020). https://doi.org/10.1021/acsmaterialslett.0c00358
L. Huang, X. N. Li, Y. Shen, R. H. Song, W. B. Cui, and H. Zhang, Dalton Trans. 53, 5192 (2024). https://doi.org/10.1039/d3dt03647b
K. J. Li, Y. Shen, S. L. Li, and X. M. Zhang, Dyes Pigm. 224, 112033 (2024). https://doi.org/10.1016/j.dyepig.2024.112033
W. G. Santos, D. S. Budkina, P. F. G. M. da Costa, D. R. Cardoso, A. N. Tarnovsky, and M. D. E. Forbes, Mater. Adv. 3, 3862 (2022). https://doi.org/10.1039/d1ma01030a
J. Ying, L. Jin, C. X. Sun, A. X. Tian, and X. L. Wang, Chem. Eur. J. 27, 1 (2021). https://doi.org/10.1002/chem.202103268
C. M. Yu, P. H. Wang, Q. Liu, L. Z. Cai, and G. C. Guo, Cryst. Growth Des. 21, 1323 (2021). https://doi.org/10.1021/acs.cgd.0c01597
B. Chen, Y. R. Huang, K. Y. Song, X. L. Lin, H. H. Li, and Z. R. Chen, Chem. Mater. 33, 2178 (2021). https://doi.org/10.1021/acs.chemmater.1c00090
M. Y. Guo, G. Li, S. L. Yang, R. Bu, X. Q. Piao, and E. Q. Gao, Chem. Eur. J. 27, 16415 (2021). https://doi.org/10.1002/chem.202102413
Y. W. Wang, M. H. Li, S. Q. Zhang, X. Fang, and M. J. Lin, Cryst. Eng. Comm. 23, 6267 (2021). https://doi.org/10.1039/d1ce00789k
Z. H. Li, L. P. Xue, Y. Wu, Q. Wang, and B. T. Zhao, Dyes Pigm. 195, 109743 (2021). https://doi.org/10.1016/j.dyepig.2021.109743
G. Zhao, W. Liu, F. Yuan, and J. Liu, Dyes Pigm. 188, 109196 (2021). https://doi.org/10.1016/j.dyepig.2021.109196
L. Liu, Q. Liu, R. Li, M. S. Wang, and G. C. Guo, J. Am. Chem. Soc. 143, 2232 (2021). https://doi.org/10.1021/jacs.0c10183
V. V. Avdeeva, A. S. Kubasov, S. E. Nikiforova, L. V. Goeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 68, 1406 (2023). https://doi.org/10.1134/S0036023623601794
X. Yang, C. Yan, Z. Li, X. Li, Q. Yu, T. Sang, Y. Gai, Q. Zhang, and K. Xiong, Inorg. Chem. 60, 5988 (2021). https://doi.org/10.1021/acs.inorgchem.1c00404
J. J. Liu, Z. L. Sun, J. Liu, and S. B. Xia, J. Mol. Struct. 1238, 130444 (2021). https://doi.org/10.1016/j.molstruc.2021.130444
M. Zuo, Y. Liu, N. Yuan, Y. Gao, Y. Li, Y. Ma, M. Sun, and S. Cui, J. Solid State Chem. 307, 122868 (2022). https://doi.org/10.1016/j.jssc.2021.122868
C. Sun, C. Yang, P. X. Li, M. S. Wang, and G. C. Guo, Inorg. Chem. 60, 9278 (2021). https://doi.org/10.1021/acs.inorgchem.1c01521
K. Q. Hu, L. W. Zeng, X. H. Kong, Z. W. Huang, J. P. Yu, L. Mei, Z. F. Chai, and W. Q. Shi, Eur. J. Inorg. Chem. 2021, 5077 (2021). https://doi.org/10.1002/ejic.202100819
G. M. Li, Z. G. Liang, Z. Z. Xue, S. D. Han, J. Pan, and G. M. Wang, Dalton Trans. 51, 4310 (2022). https://doi.org/10.1039/d2dt00190j
ACKNOWLEDGMENTS
This project was financially supported by the Natural Science Foundation of Jilin Province (YDZJ202101ZYTS067, YDZJ202101ZYTS072), Education Department Foundation of Jilin Province (JJKH20220045KJ).
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Ran Tao, Wang, X., Wang, X. et al. A Novel Multichromic Cd(II) Coordination Polymer Based on a Viologen Ligand Exhibiting Multichroism, Aniline Detection and Luminescence Properties. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023624601119
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023624601119