Abstract
The phase relationships in the quasi-quaternary system GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 at 1373 K in air have been studied. The homogeneity ranges and crystal structure of solid solutions with overall composition Gd1–xSrxCo1–yFeyO3–δ have been determined. Depending on the concentration of introduced strontium and iron, the Gd1–xSrxCo1–yFeyO3–δ oxides crystallize in orthorhombic (x = 0.1 and 0.4 ≤ y ≤ 1.0; x = 0.2 and y = 0.9, space group Pbnm), tetragonal (0.6 ≤ x ≤ 0.8 and 0.1 ≤ y ≤ 0.5, space group I4/mmm) or cubic (x = 0.9 and 0.1 ≤ y ≤ 0.9; 0.6 ≤ x ≤ 0.8 and 0.6 ≤ y ≤ 0.9, space group Pm\(\overline{3}\)m) perovskite structure. Structural parameters were determined for all single-phase samples. It was found that an increase in the concentration of strontium and iron leads to an increase in the unit cells parameters of the Gd1–xSrxCo1–yFeyO3–δ oxides. It has been shown that the oxygen content in the Gd1–xSrxCo0.3Fe0.7O3–δ oxides, determined by thermogravimetric analysis, decreases with increasing temperature and strontium content. An isobaric-isothermal phase diagram for the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system at 1373 K in air was constructed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The set of interesting physicochemical characteristics of complex oxides with a perovskite structure, in which rare earth and alkaline earth elements are located in the A-sites, and cobalt and iron in the B‑sites [1–5], open up prospects for their practical use in a variety of electrochemical and catalytic devices. The study of the crystal structure and physicochemical properties of strontium-substituted cobaltites Ln1‒xSrxCoO3–δ (Ln = REE) was undertaken due to the possibility of their practical use as cathodes [6–12] or interconnectors [13] for intermediate-temperature solid oxide fuel cells (SOFCs), cathodes of CO2 lasers [14], catalysts [15–17], gas-sensitive sensors [16, 18, 19], materials for chemical looping [20], thermoelectrics [21], magnetic materials [22–25].
Depending on the radius of the lanthanide ion, the concentration of introduced strontium, and the heat treatment conditions, the Ln1–xSrxCoO3–δ oxides can crystallize in the rhombohedral (Ln = La with 0.0 ≤ x ≤ 0.5, space group R\(\overline{3}\)c) [26–29], orthorhombic (Ln = Pr, Nd, Sm with 0.0 ≤ x ≤ 0.6 and Ln = Gd with 0.37 ≤ x ≤ 0.6 space group Pbnm or Pnma) [8, 26, 30–32] or cubic Ln = La with 0.6 ≤ x ≤ 0.8 (space group Pm\(\overline{3}\)m) [28, 29] perovskite structure. Cobaltites Ln1‒xSrxCoO3–δ enriched with strontium 0.6 < x < 0.9 are characterized by structural ordering of lanthanide and strontium ions in the A-sublattice, leading to the localization of oxygen vacancies in certain planes and the formation of a tetragonal superstructure ap × ap × 2ap for Ln = La, Pr, Nd (space group P4/mmm) and 2ap × 2ap × 4ap for Ln = Sm, Gd, Dy, Y, Ho (space group I4/mmm) [26, 30, 33–36]. An increase in temperature leads to the destruction of superstructural ordering, and the phase transition temperature depends on the Ln/Sr ratio [26, 34–37]. For example, in air the transition from an ordered 2ap × 2ap × 4ap superstructure to a disordered cubic one with statistically distributed Ln and Sr cations in the A-sublattice in Gd0.2Sr0.8CoO3–δ is completed at 1363 K [36], which is in good agreement with the results in [37]. A similar structural transition in Gd0.1Sr0.9CoO3–δ in air occurs at 1263 K [38]. A slightly different interpretation, although similar from the crystallographic point of view of the formation of an ordered arrangement of cations in the A-sublattice with the formation of a tetragonal cell, was given by Istomin et al. [39].
Oxygen nonstoichiometry in the Ln1–xSrxCoO3–δ (Ln = La–Gd) oxides changes slightly with decreasing radius of the lanthanide ion and increases significantly with increasing strontium concentration in the samples [7, 26, 28, 30, 32, 37, 40]. It should also be noted that the oxygen content, along with the Ln/Sr ratio, significantly determines the formation of a superstructure in cobaltites [32, 41].
The introduction of iron into the cobalt sublattice in Ln1–xSrxCoO3–δ has a noticeable effect on the crystal structure and the entire set of physicochemical properties of the Ln1–xSrxCo1–yFeyO3–δ (Ln = La–Gd) oxides [42–50]. Information on the structure and properties of solid solutions based on GdCoO3 with simultaneous substitution in the A- and B-sublattices is scarce. It is known that cobaltites Gd0.8Sr0.2Co1–y-FeyO3–δ (0.0 ≤ y ≤ 1.0) crystallize in an orthorhombic perovskite-like structure (space group Pbnm), and an increase in the iron concentration in the samples leads to a decrease in the thermal expansion coefficients and electrical conductivity [42, 43, 49]. This work is devoted to the study of phase relations in the quasi-quaternary GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system at 1373 K in air and influence of the substitution value on the homogeneity range, crystal structure and oxygen nonstoichiometry of Gd1–xSrxCo1–yFeyO3–δ complex oxides.
EXPERIMENTAL
The synthesis of samples was carried out using glycerol-nitrate technique, described earlier in [45, 50]. The starting components were gadolinium oxide Gd2O3 (99.99%), strontium carbonate SrCO3 (high purity grade), pre-calcined to remove adsorbed moisture and gases at 1373 K for 12 h and 773 K for 5 h, respectively, iron oxalate FeC2O4⋅2H2O (analytical grade) and metal cobalt Co, obtained by reduction from cobalt oxide Co3O4 (analytical grade) at 673–873 K in a hydrogen flow. Final annealing was carried out at 1373 K in air for 60–80 h with intermediate grinding in ethyl alcohol after 12–15 h. The samples were quenched in air from 1373 K to room temperature by removing crucibles with a volume of <1 mL from a heated furnace onto a massive copper plate (cooling rate ∼400–500 deg/min).
The phase composition of the studied samples was determined by X-ray powder diffraction using a Shimadzu XRD 7000 diffractometer with CuKα radiation (λ = 1.5418 Å) using a pyrolytic graphite monochromator (angle range 10° ≤ 2θ ≤ 80°, step 0.02°, exposure at point 2 s). The structural parameters were refined using the Rietveld full-profile analysis method in the Fullprof-2011 software.
High-temperature XRD (HT-XRD) studies were carried out using an Inel Equinox 3000 diffractometer equipped with an HTK 16N high-temperature chamber (Anton Paar). Heating and cooling of the sample to a required temperature was performed at a rate of 100 K/h. The accuracy of temperature maintenance was ± 0.1 K.
The oxygen content in the oxides and its change with temperature were determined by thermogravimetric analysis. Measurements of temperature dependences were carried out using a thermal analyzer STA 409 PC Luxx in dynamic mode with a heating/cooling rate of 2 K/min in the temperature range 298–1373 K in air. The absolute value of oxygen content in oxides was determined by a complete reduction of samples with hydrogen (10% N2 + 90% H2) at 1423 K in a thermogravimetric cell to oxides Gd2O3, SrO and metallic cobalt Co and iron Fe. The phase composition of the reduced samples was controlled by X-ray powder diffraction.
RESULTS AND DISCUSSION
To study phase equilibria in the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system 52 samples with various ratios of metal components were prepared at 1373 K in air using glycerol-nitrate technique.
Crystal Structure of Gd1–xSrxCo1–yFeyO3–δ Oxides
Using X-ray powder diffraction, it was established that the homogeneity ranges and crystal structure of the Gd1–xSrxCo1–yFeyO3–δ solid solutions depend significantly on the concentration of introduced strontium (x) and iron (y).
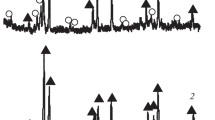
X-ray powder diffraction patterns of single-phase Gd1–xSrxCo1–yFeyO3–δ oxides with x = 0.0 and 0.0 ≤ y ≤ 1.0, x = 0.1 and 0.4 ≤ y ≤ 1.0 and x = 0.2 and y = 0.9 quenched from 1373 K in air, similar to unsubstituted GdMeO3 (Me = Fe, Co), were indexed within an orthorhombically distorted perovskite-like cell (space group Pbnm). Figure 1 shows, as an example, diffraction patterns of Gd1–xSrxCo0.2Fe0.8O3–δ with x = 0.0 and 0.1, refined using the Rietveld full-profile analysis. The refined unit cell parameters of Gd1–xSrx-Co1–yFeyO3–δ (x = 0.0; 0.1) are listed in Table 1. The linear increase in the unit cell parameters and unit cell volume for Gd1–xSrxCo1–yFeyO3–δ (x = 0.0; 0.1) with increasing iron content (Fig. 2) corresponds to a larger radius of iron ions ((\(r_{{{\text{Fe}}}}^{{{\text{3 + }}}}\)/\(_{{{\text{Fe}}}}^{{{\text{4 + }}}}\)(HS) = 0.645/0.585 Å, CN = 6) compared to the radius of cobalt ions (\(r_{{{\text{Co}}}}^{{{\text{3 + }}}}\)/\(_{{{\text{Co}}}}^{{{\text{4 + }}}}\) (HS) = 0.61/0.53 Å, CN = 6) [51].
X-ray powder diffraction data for Gd1–xSrxCo0.2Fe0.8O3–δ with x = 0.0 (a) and x = 0.1 (b), refined using the Rietveld method. Points show experimental data; (1) theoretical spectrum; (2) positions of peaks with allowed Miller indices (hkl); (3) difference between experimental data and theoretical curve.
The homogeneity range of Gd1–xSrxCo1–yFeyO3–δ solid solutions with an orthorhombic structure is significantly smaller than that determined for similar oxides in the Nd-containing system Nd1–xSrxCo1–y-FeyO3–δ [45]. This can be explained by the larger difference in radii of gadolinium (\(r_{{{\text{Gd}}}}^{{{\text{3 + }}}}\) = 1.107 Å) and strontium (\(r_{{{\text{Sr}}}}^{{{\text{2 + }}}}\) = 1.44 Å) compared to the difference in radii between neodymium (\(r_{{{\text{Nd}}}}^{{{\text{3 + }}}}\) = 1.27 Å) and strontium [51], and as a result, the replacement of Gd by Sr from the side of GdCoO3 does not occur at all [52], and from the GdFeO3 side it is significantly less [53].
An increase in the concentration of strontium, which replaces gadolinium in Gd1–xSrxCo1–yFeyO3–δ up to x = 0.6, again leads to the formation of single-phase oxides and a change in their crystallographic symmetry. Sr-enriched cobaltites Gd1–xSrxCo1–y-FeyO3–δ with 0.6 ≤ x ≤ 0.9, depending on the iron content, have a tetragonal (space group I4/mmm) or cubic unit cell (space group Pm\(\overline{3}\)m). In the tetragonal ordered 2ap × 2ap × 4ap structure, REE and strontium cations are distributed over three nonequivalent positions: A1 is completely occupied by REE ions, A2 occupied by strontium ions, and both REE and Sr are located in A3 positions [26, 38, 54]. Structural transitions in Gd1–xSrxCo1–yFeyO3–δ with x = 0.8 and 0.9 were described in detail in our previous work [55].
The X-ray powder diffraction patterns of the Gd1–x-SrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.7 and 0.1 ≤ y ≤ 0.5, quenched at 1373 K in air, similar to iron-free cobaltites Gd1–xSrxCoO3–δ (0.6 ≤ x ≤ 0.8) [26, 30, 37, 39, 52] contain superstructure reflections at scattering angles of 2θ ≈ 21° (d ≈ 4.26 Å, hkl = 103) and 2θ ≈ 39° (d ≈ 2.29 Å, hkl = 215), indicating the formation of a tetragonal supercells 2ap × 2ap × 4ap (where ap is the unit cell parameter of the basic perovskite). According to electron diffraction data [26, 30, 33, 39], the superstructure (2ap × 2ap × 4ap) is formed by ordering of Gd and Sr cations in the A sublattice and the accompanying ordered arrangement of oxygen vacancies.
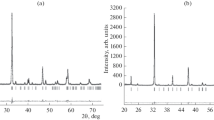
X-ray powder diffraction patterns of the Gd1‒xSrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.7 and 0.1 ≤ y ≤ 0.5 were indexed in the tetragonal cell 2ap × 2ap × 4ap (space group I4/mmm). Figure 3a, as an example, shows a typical X-ray powder diffraction pattern of Gd0.3Sr0.7Co0.8Fe0.2O3–δ cobaltite refined by the Rietveld full-profile method. The structural parameters for Gd1–xSrxCo1–yFeyO3–δ (0.6 ≤ x ≤ 0.7 and 0.1 ≤ y ≤ 0.5) oxides refined by the Rietveld method are presented in Tables 2 and 3. The unit cell parameters and atomic coordinates for the Gd0.2Sr0.8Co1–yFeyO3–δ (0.1 ≤ y ≤ 0.5) solid solutions with a tetragonal structure are given in [55]. Substitution of Gd3+ (r = 1.107 Å) with larger Sr2+ cations (r = 1.44 Å) [51] and an increase in the content of iron replacing cobalt in Gd1–xSrxCo1–yFeyO3–δ (0.6 ≤ x ≤ 0.7) from y = 0.1 to y = 0.5 lead to an increase in the unit cell parameters and volume, which is caused by the size effect (Fig. 4).
X-ray powder diffraction data for Gd0.3Sr0.7Co1–y-FeyO3–δ with y = 0.2 (a) and y = 0.7 (b), refined using the Rietveld method. Points show experimental data; (1) theoretical spectrum; (2) positions of peaks with allowed Miller indices (hkl); (3) difference between experimental data and theoretical curve. Arrows indicate superstructural reflections for the tetragonal cell 2ap × 2ap × 4ap.
One of the most important factors in the formation of a superstructure with an ordered arrangement of cations in A-positions is the Gd/Sr ratio [26, 54], therefore, the replacement of Co by Fe, which is similar in many properties, does not initially affect the type of structure. However, along with the ordering of cations, the ordering of oxygen vacancies also plays an important role in the formation of the superstructure, and the introduction of iron ions, although not to the same extent as the replacement of Gd by Sr, still changes the oxygen content. An increase in the iron content in the Gd1–xSrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.7 up to y = 0.6 leads to a transition from a tetragonal superstructure, with ordered Gd and Sr cations in the A-sublattice, to a cubic structure, with statistically distributed cations. X-ray powder diffraction patterns of the Gd1–xSrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.7 and 0.6 ≤ y ≤ 0.9, like those for the Gd1–xSrxFeO3–δ ferrites, were indexed in an ideal cubic structure (space group Pm\(\overline{3}\)m) [53]. Figure 3b, as an example, shows the X-ray powder diffraction pattern of cubic Gd0.3Sr0.7Co0.3Fe0.7O3–δ, refined by the Rietveld full-profile method. The structural parameters of Gd1‒xSrxCo1–yFeyO3–δ solid solutions with a cubic structure are listed in Table 4.
To compare the unit cell parameters of the Gd1‒xSrxCo1–yFeyO3–δ oxides with x = 0.6 and 0.7 over the entire concentration range (0.1 ≤ y ≤ 0.9), the parameters of the tetragonal supercell were recalculated to the pseudocubic one using the formula:
where V is a volume of tetragonal supercell, z is the number of formula units (for 2ap × 2ap × 4ap supercells z = 16). It can be seen that the dependence of the pseudocubic cell parameters (acubic) for the Gd1‒xSrxCo1–yFeyO3–δ oxides (x = 0.6 and 0.7) versus iron concentration (Fig. 5) exhibits a discontinuity in the composition range 0.5 ≤ y ≤ 0.6 corresponding to the “order–disorder”-type structural transition, similar to that reported for cobaltites Gd1–xSrxCoO3–δ with increasing strontium content [37].
The X-ray powder diffraction patterns of samples with the nominal composition of Gd1–xSrxCo1–yFeyO3–δ with 0.2 ≤ x ≤ 0.5 and 0.2 ≤ y ≤ 0.8 contained reflections of two types of solid solutions with orthorhombic (space group Pbnm) and cubic (space group Pm\(\overline{3}\)m) structures.
Oxygen Nonstoichiometry in Gd1–xSrxCo1–yFeyO3–δ Oxides
The temperature dependences of the oxygen content in Gd1–xSrxCo0.3Fe0.7O3–δ solid solutions with 0.6 ≤ x ≤ 0.9 are shown in Fig. 6. It can be seen that oxygen content in the samples noticeably decreases with increasing strontium concentration (Table 5). Heterovalent substitution of strontium for gadolinium leads to the formation of acceptor-type defects \({\text{Sr}}^{'}_{\text{Gd}}\). Compensation for the excess negative charge in the structure occurs through the formation of an equivalent number of positively charged oxygen vacancies (\(V_{\text{O}}^{{\bullet \bullet }}\)) and holes (\(h\bullet \)) localized on 3d transition metal ions. With increasing strontium content, the average oxidation state (Z) of 3d transition metal ions changes slightly from 3.02+ for x = 0.6 to 3.08 + for x = 0.9 at 1373 K (ΔZ = 0.06) compared to the change in oxygen content from 2.71 for x = 0.6 to 2.59 for x = 0.9 at 1373 K (Δ(3 – δ) = 0.12, which corresponds to charge compensation ΔZ = 0.24) (see Table 5). Thus, the lability of the oxygen sublattice [56] leads to the formation of solid solutions within a fairly wide range, since the stability of oxides with a perovskite structure is largely determined by the thermodynamically favorable oxidation state of 3d metals at certain T, Po2 [57], and charge compensation in Gd1–xSrxCo0.3Fe0.7O3–δ within the studied temperature range in air is determined mainly due to the formation of oxygen vacancies (\(V_{\text{O}}^{{\bullet \bullet }}\)).
In a case when the average oxidation state of 3d metals is above 3+, the more electropositive iron ions will predominantly have oxidation state of 4+ (electronegativity of Fe = 1.64; electronegativity of Co = 1.7 [58]). In other words, the equilibrium in the reaction Fe4+ + Co3+ = Fe3+ + Co4+ is significantly shifted to the left-hand side. Using the electroneutrality condition and experimentally determined values of oxygen content, the formulas of solid solutions Gd1–x-SrxCo0.3Fe0.7O3–δ can be represented as follows:
T = 298 K, air | T = 1373 K, air |
|---|---|
\({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.4}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.6}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.22}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.48}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.94}}}}}\) | \({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.4}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.6}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.68}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.02}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.71}}}}}\) |
\({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.3}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.7}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.24}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.46}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.88}}}}}\) | \({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.3}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.7}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.66}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.04}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.67}}}}}\) |
\({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.2}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.8}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.22}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.48}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.84}}}}}\) | \({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.2}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.8}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.62}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.08}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.64}}}}}\) |
\({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.1}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.9}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.18}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.52}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.81}}}}}\) | \({\text{G}}{{{\text{d}}}_{{{\text{0}}{\text{.1}}}}}{\text{S}}{{{\text{r}}}_{{{\text{0}}{\text{.9}}}}}{\text{Co}}_{{{\text{0}}{\text{.3}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.64}}}}^{{{\text{3}} + }}{\text{Fe}}_{{{\text{0}}{\text{.06}}}}^{{{\text{4}} + }}{{{\text{O}}}_{{{\text{2}}{\text{.59}}}}}\) |
It can be seen that although the charge compensation with increasing strontium concentration is carried out mainly due to the release of oxygen from the lattice which is accompanied with the formation of oxygen vacancies, the fraction of Fe4+ ions also increase slightly. Similar results were obtained earlier for perovskite-like oxides of the composition Ln1–xSrxCo1–y-FeyO3–δ (Ln = La, Nd) [44, 45, 50].
Phase Diagram of the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 System
The phase diagrams of quasi-binary systems GdCoO3–SrCoO3–δ, GdFeO3–SrFeO3–δ, SrCoO3‒δ–SrFeO3–δ necessary for constructing the phase diagram of the quasi-quaternary system GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 at 1373 K in air, were studied in detail earlier in [52, 53, 59]. The homogeneity range limits and the type of crystal structure for the oxides formed in these systems at 1373 K in air are given in Table 6.
The X-ray powder diffraction pattern of SrCoO3–δ cobaltite quenched from 1373 K in air was indexed within an orthorhombic cell with unit cell parameters a = 5.615(1) Å, b = 15.580(1) Å, c = 5.563(1) Å (space group Pnma) [59], which is in good agreement with the results reported in [60]. However, according to HT-XRD data [52], SrCoO3–δ at 1373 K in air crystallizes in an ideal cubic structure (space group Pm\(\overline{3}\)m), and the quenching rate after removing the sample to room temperature is insufficient to preserve the structure. To clarify the homogeneity range of the SrFe1‒xCoxO3–δ solid solution, HT-XRD measurement of the SrFe0.2Co0.8O3–δ complex oxide was additionally performed. According to the obtained results cobaltite SrFe0.2Co0.8O3–δ at 1373 K in air possesses a cubic perovskite structure with the unit cell parameter a = 3.984(1) Å (space group Pm\(\overline{3}\)m). Thus, in the SrCoO3–δ–SrFeO3–δ system under experimental conditions studied a series of solid solutions SrFe1‒xCoxO3–δ with a tetragonal structure is formed inside the range 0.0 ≤ x < 0.3, and another one with a cubic structure inside the range 0.3 ≤ x ≤ 1.0, which is consistent with the data reported in [61].
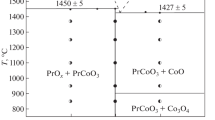
Taking into account the X-ray powder diffraction results of all studied samples quenched to room temperature, a phase diagram for the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system at 1373 K in air was constructed (Fig. 7). The phase diagram shows the stability regions of Gd1–xSrxCo1–yFeyO3–δ solid solutions with orthorhombic (space group Pbnm), tetragonal (space group I4/mmm) and cubic (space group Pm\(\overline{3}\)m) structure. The structural transition of “order⇔disorder”-type for the Gd1–xSrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.8 at y > 0.5 refers to the second order phase transitions, which boundary is extended and cannot be attributed to fixed parameters, thus in the phase diagram it is shown by a dash line. The shaded fields correspond to the coexistence of two types of solid solutions.
Isobaric-isothermal phase diagram for the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system at 1373 K in air. Green points correspond to the orthorhombic structure of Gd1–xSrxCo1–yFeyO3–δ (space group Pbnm), blue points to the cubic structure (space group Pm-3m), red points to the tetragonal ordered structure (space group I4/mmm). Purple dots correspond to the two-phase region where orthorhombic and tetragonal (or cubic) structures coexist.
CONCLUSIONS
It has been shown that the crystal structure of Gd1–x-SrxCo1–yFeyO3–δ solid solutions depends significantly on the concentration of introduced strontium and iron. At low strontium contents, Gd1–xSrxCo1–yFeyO3–δ complex oxides (x = 0.0 and 0.0 ≤ y ≤ 1.0; x = 0.1 and 0.4 ≤ y ≤ 1.0; x = 0.2 and y = 0.9) have an orthorhombically distorted perovskite structure (space group Pbnm); strontium-enriched Gd1–xSrxCo1–yFeyO3–δ oxides with 0.6 ≤ x ≤ 0.8 and low iron content of 0.1 ≤ y ≤ 0.5 crystallize in a tetragonal supercell 2ap × 2ap × 4ap (space group I4/mmm) with ordered Gd and Sr cations in the A-sublattice, and oxides with 0.6 ≤ x ≤ 0.8 and high iron content 0.6 ≤ y ≤ 0.9 crystallize in a cubic cell with statistically distributed cations. The increase in the unit cell parameters of the Gd1‒xSrxCo1–yFeyO3–δ oxides with increasing strontium and/or iron content is associated with the size effect. The oxygen content in Gd1–xSrxCo1–yFeyO3–δ cobaltites decreases with increasing temperature (which corresponds to more reducing conditions), increasing strontium concentration (representing the acceptor-type substituent \({\text{Sr}}^{'}_{\text{Gd}}\)) and, to a slightly lesser extent, with decreasing iron concentration (representing the donor-type substituent). An isobaric-isothermal section of the phase diagram of the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 system at 1373 K in air has been constructed.
REFERENCES
A. I. Klyndyuk, Ya. Yu. Zhuravleva, N. N. Gundilovich, et al., Inorg. Mat. 59, 86 (2023). https://doi.org/10.1134/S0020168523010089
T. L. Simonenko, N. P. Simonenko, E. P. Simonenko, et al., Russ. J. Inorg. Chem. 67, 1495 (2022). https://doi.org/10.1134/S0036023622600939
A. I. Klyndyuk and Ya. Yu. Zhuravleva, Russ. J. Inorg. Chem. 67, 2084 (2022). https://doi.org/10.1134/S0036023622601404
M. V. Kalinina, D. A. Dyuskina, I. G. Polyakova, et al., Glass Phys. Chem. 49, 177 (2023). https://doi.org/10.1134/S1087659622601046
E. A. Chizhova, A. I. Klyndyuk, Ya. Yu. Zhuravleva, et al., Glass Phys. Chem. 49, 57 (2023). https://doi.org/10.1134/S1087659622600910
H. Fan, Z. Liu, Y. Wu, et al., Int. J. Appl. Ceram. Technol. 21, 289 (2023). https://doi.org/10.1111/ijac.14490
K. T. Lee and A. Manthiram, J. Electrochem. Soc. 153, A794 (2006). https://doi.org/10.1149/1.2172572
K. T. Lee and A. Manthiram, J. Electrochem. Soc. 152, A197 (2005). https://doi.org/10.1149/1.1828243
C. Rossignol, J. M. Ralph, J.-M. Bae, et al., Solid State Ionics 175, 59 (2004). https://doi.org/10.1016/j.ssi.2004.09.021
Y. Takeda, H. Ueno, N. Imanishi, et al., Solid State Ionics 86–88, 1187 (1996). https://doi.org/10.1016/0167-2738(96)00285-8
Q. Ni, H. Chen, L. Ge, et al., J. Power Sources 349, 130 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.037
X. Tong, S. Ovtar, K. Brodersen, et al., J. Power Sources 451, 227742 (2020). https://doi.org/10.1016/j.jpowsour.2020.227742
R. K. Madathil and T. Norby, Solid State Sci. 124, 106801 (2022). https://doi.org/10.1016/j.solidstatesciences.2021.106801
O. F. Kononchuk, A. N. Petrov, and V. A. Cherepanov, Inorg. Mater. 27, 1662 (1991).
S. N. Vereshchagin, L. A. Solovyov, E. V. Rabchevskii, et al., Chem. Commun. 50, 6112 (2014). https://doi.org/10.1039/c4cc00913d
C. Tealdi, IslamM. Saiful, C. Fisher, et al., Prog. Solid State Chem. 35, 491 (2007). https://doi.org/10.1016/j.progsolidstchem.2007.01.015
X. Wang, K. Huang, W. Ma, et al., Chem.-Eur. J. 23, 1093 (2017). https://doi.org/10.1002/chem.201604065
H. Liu, Y. Guo, R. Xie, et al., Sens. Actuat. B 246, 164 (2017). https://doi.org/10.1016/j.snb.2017.02.072
J. He, J. Sunarso, J. Miao, et al., J. Hazard. Mater. 369, 699 (2019). https://doi.org/10.1016/j.jhazmat.2019.02.070
T. Li, R. S. Jayathilake, D. D. Taylor, et al., Chem. Commun. 55, 4929 (2019). https://doi.org/10.1039/C8CC09573F
V. A. Dudnikov, Y. S. Orlov, N. V. Kazak, et al., Ceram. Int. 45, 5553 (2019). https://doi.org/10.1016/j.ceramint.2018.12.013
M. S. Reis, D. L. Rocco, VivasR. J. Caraballo, et al., J. Magn. Magn. Mater. 422, 197 (2017). https://doi.org/10.1016/j.jmmm.2016.08.080
K. H. Ryu, K. S. Roh, S. J. Lee, et al., J. Solid State Chem. 105, 550 (1993). https://doi.org/10.1006/jssc.1993.1247
L. Zhang, X. Li, F. Wang, et al., Mater. Res. Bull. 48, 1088 (2013). https://doi.org/10.1016/j.materresbull.2012.11.105
P. T. Long, T. V. Manh, T. A. Ho, et al., Ceram. Int. 44, 15542 (2018). https://doi.org/10.1016/j.ceramint.2018.05.216
M. James, D. Cassidy, D. J. Goossens, et al., J. Solid State Chem. 177, 1886 (2004). https://doi.org/10.1016/j.jssc.2004.01.012
N. Alhokbany, S. Almotairi, J. Ahmed, et al., J. King Saud. Univer. Sci. 33, 101419 (2021). https://doi.org/10.1016/j.jksus.2021.101419
A. N. Petrov, O. F. Kononchuk, A. V. Andreev, et al., Solid State Ionics 80, 189 (1995). https://doi.org/10.1016/0167-2738(95)00114-l
V. A. Cherepanov, L. Ya. Gavrilova, L. Yu. Barkhatova, et al., Ionics 4, 309 (1998). https://doi.org/10.1007/BF02375959
M. James, T. Tedesco, D. J. Cassidy, et al., Mater. Res. Bull. 40, 990 (2005). https://doi.org/10.1016/j.materresbull.2005.02.020
S. Park, S. Choi, J. Shin, et al., J. Power Sources 210, 172 (2012). https://doi.org/10.1016/j.jpowsour.2012.03.018
T. V. Aksenova, T. G. Efimova, O. I. Lebedev, et al., J. Solid State Chem. 248, 183 (2017). https://doi.org/10.1016/j.jssc.2017.02.002
M. James, M. Avdeev, P. Barnes, et al., J. Solid State Chem. 180, 2233 (2007). https://doi.org/10.1002/chin.200835004
V. A. Dudnikov, Y. S. Orlov, N. V. Kazak, et al., Ceram. Int. 44, 10299 (2018). https://doi.org/10.1016/j.ceramint.2018.03.037
S. N. Vereshchagin, V. A. Dudnikov, N. N. Shishkina, et al., Thermochim. Acta 655, 34 (2017). https://doi.org/10.1016/j.tca.2017.06.003
V. A. Dudnikov, Yu. S. Orlov, S. Yu. Gavrilkin, et al., J. Phys. Chem. 120, 13443 (2016). https://doi.org/10.1021/acs.jpcc.6b04810
A. V. Maklakova, A. S. Baten’kova, M. A. Vlasova, et al., Solid State Sci. 110, 106453 (2020). https://doi.org/10.1016/j.solidstatesciences.2020.106453
V. A. Dudnikov, N. V. Kazak, Y. S. Orlov, et al., J. Exp. Theor. Phys. 128, 630 (2019). https://doi.org/10.1134/S1063776119020171
S. Y. Istomin, O. A. Drozhzhin, and G. Svensson, Solid State Sci. 6, 539 (2004). .https://doi.org/10.1016/j.solidstatesciences.2004.03.029
A. N. Petrov, V. A. Cherepanov, O. F. Kononchuk, et al., J. Solid State Chem. 87, 69 (1990). https://doi.org/10.1016/0022-4596(90)90066-7
M. James, L. Morales, K. Wallwork, et al., Physica B 385–386, 199 (2006). https://doi.org/10.1016/j.physb.2006.05.244
L. Qiu, T. Ichikawa, A. Hirano, et al., Solid State Ionics 158, 55 (2002). https://doi.org/10.1016/S0167-2738(02)00757-9
C. R. Dyck, G. Yu, and V. D. Krstic, Mat. Res. Soc. Symp. Proc. 801, 114 (2003). https://doi.org/10.1557/PROC-801-BB3.4
T. V. Aksenova, V. A. Cherepanov, L. Ya. Gavrilova, et al., Prog. Solid State Chem. 35, 175 (2007). https://doi.org/10.1016/j.progsolidstchem.2007.03.001
Sh. I. Elkalashy, A. R. Gilev, T. V. Aksenova, et al., Solid State Ionics 31, 85 (2018). https://doi.org/10.1016/j.ssi.2017.12.028
Q. Xu, D. Huang, W. Chen, et al., J. Alloys Compd. 429, 34 (2007). https://doi.org/10.1016/j.jallcom.2006.04.005
N. Dasgupta, R. Krishnamoorthy, and J. K. Thomas, Mater. Sci. Eng. 90, 278 (2002). https://doi.org/10.1016/S0921-5107(02)00058-2
K. T. Lee and A. Manthiram, Solid State Ionics 176, 1521 (2005). https://doi.org/10.1016/j.ssi.2005.05.002
F. Riza, Ch. Ftikos, F. Tietz, et al., J. Eur. Ceram. Soc. 21, 1769 (2001). https://doi.org/10.1016/S0955-2219(01)00112-1
Sh. I. Elkalashy, T. V. Aksenova, A. S. Urusova, et al., Solid State Ionics 295, 96 (2016). https://doi.org/10.1016/j.ssi.2016.08.005
R. D. Shannon, Acta Crystallogr. A 32, 751 (1976). https://doi.org/10.1107/S0567739476001551
A. V. Maklakova, M. A. Vlasova, N. E. Volkova, et al., J. Alloys Compd. 883, 160794 (2021). https://doi.org/10.1016/j.jallcom.2021.160794
L. V. Khvostova, N. E. Volkova, L. Ya. Gavrilova, et al., Mater. Today Comm. 29, 102885 (2021). https://doi.org/10.1016/j.mtcomm.2021.102885
N. E. Volkova, A. V. Maklakova, L. Ya. Gavrilova, et al., Eur. J. Inorg. Chem. 2017, 3285 (2017). https://doi.org/10.1002/ejic.201700321
T. V. Aksenova, D. K. Mysik, and V. A. Cherepanov, Catalysts 12, 1344 (2022). https://doi.org/10.3390/catal12111344
E. V. Tsipis, E. N. Naumovich, M. V. Patrakeev, et al., J. Solid State Electrochem. 25, 2777 (2021). https://doi.org/10.1007/s10008-021-05023-8
V. A. Cherepanov, L. Yu. Barkhatova, and A. N. Petrov, J. Phys. Chem. Solids 55, 229 (1994). https://doi.org/10.1016/0022-3697(94)90137-6
J. I. Huheey, Inorganic Chemistry (Harper & Row, New York, 1983).
T. V. Aksenova, L. Ya. Gavrilova, and V. A. Cherepa-nov, J. Solid State Chem. 10, 1480 (2008). https://doi.org/10.1016/j.jssc.2008.03.010
J. C. Grenier, L. Fournes, M. Pouchard, et al., Mater. Res. Bull. 21, 441 (1986). https://doi.org/10.1016/0025-5408(86)90009-7
T. Takeda and H. Watanabe, J. Phys. Soc. Jpn. 33, 973 (1972). https://doi.org/10.1143/JPSJ.33.973
Funding
The work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (state registration number 123031300049-8).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aksenova, T.V., Solomakhina, E.E., Urusova, A.S. et al. Phase Equilibria, Crystal Structures, and Oxygen Nonstoichiometry of Complex Oxides Formed in the GdCoO3–SrCoO3–δ–SrFeO3–δ–GdFeO3 System. Russ. J. Inorg. Chem. (2024). https://doi.org/10.1134/S0036023624601090
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S0036023624601090