Abstract
The sorption kinetics of selected metal ions from multicomponent solutions by sorbents with taurine functions based on aminopolymers, namely, on chitosan, polyallylamine, and polyethyleneimine, has been studied. Sulfoethylated polyallylamine sorbents are distinguished by the highest silver(I) sorption selectivity and the shortest equilibration time. Sulfoethylated polyethyleneimines, unlike the other sorbents studied, can be used, depending on the pH of ammonium acetate buffer solution, for the group recovery of transition-metal ions or for co-recovering silver(I), copper(II), and nickel(II). The chemical structure of the polymer matrix and the degree of its modification do not significantly affect the initial silver(I) sorption rate from multicomponent solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Aminopolymers are a class of compounds that are widely used as matrices for the synthesis of sorption materials. The presence of a large number of amino groups determines their ability to extract the target components both through ion-exchange interaction and through complex formation. The most significant change in the selectivity properties of aminopolymers is achieved by their modification with additional functional groups [1, 2]. One more important tool for varying the selectivity properties of such materials is afforded by the very nature of the aminopolymer matrix, which significantly affects the recovery of metal ions [2].
The most important property of any sorption material is the rate at which sorption equilibrium is acquired. When sorption proceeds slowly, the time required for concentration increases. When metal ion sorption rates differ significantly from one another, there is an additional opportunity for their separation [3]. This is the reason why studies into the sorption kinetics of metal ions co-present in solution are of particular interest.
Mathematical processing of the integral sorption rate curves in terms of diffusion and chemical kinetic models can help to gain important information about the mechanism and parameters of the sorption process. Although it is very difficult and sometimes incorrect to separate the contributions of diffusion and chemical stages using formal kinetic equations [4, 5], this approach is quite popular among scientists in Russia and abroad [1, 6–11]. Its popularity is partly due to the fact that a comparison of the parameters obtained by processing the corresponding dependences for various sorption materials makes it possible to reveal the influence of various factors on the metal ion sorption kinetics. At the same time, despite the sufficient amount of studies into the influence of the particle size, temperature, and other conditions of sorption experiments on the rate at which sorption equilibrium is acquired [6, 8, 9], the influence of the degree of modification and the nature of the sorbent polymer matrix on the sorption kinetics is still poorly understood.
Our prior studies concerned with the properties of some sorbents based on sulfoethylated aminopolymers, namely, chitosan [12], polyallylamine [13], and polyethyleneimine [14], with various degrees of modification, with respect to some transition and alkaline-earth metal ions that form stable complexes with taurine, the functional groups of which are contained in the considered sorbents. We found that, depending on the experimental conditions, the content of sulfoethyl groups, and the nature of the polymer matrix, the materials under study are able to selectively recover silver(I) [13] or silver(I) and copper(II) [12, 14] from multicomponent solutions or they can act as sorbents for the group recovery of selected transition-metal ions [14]. The highest silver(I) sorption selectivity is ensured when an ammonium acetate buffer solution is used, which, in addition, prevents the formation of poorly soluble species of metal ions due to the formation of stable ammoniates and makes it possible to study the recovery over a wide pH range. It is necessary to study the metal ion sorption kinetics in order to determine the prospects for using sulfoethylated aminopolymers in separation and concentration processes.
The goal of this work was to study the sorption kinetics of metal (copper(II), nickel(II), cobalt(II), zinc(II), cadmium(II), calcium(II), magnesium(II), strontium(II), barium(II), and silver(I)) ions co-present in an ammonium acetate buffer solution on aminopolymer-based sorbents, depending on the degree of modification (DM) of the sorbent by sulfoethyl groups and the nature of the matrix.
EXPERIMENTAL
Subject Matters
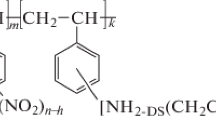
Sulfoethylated polyallylamines with a DM of 0.5 and 1.0 crosslinked with epichlorohydrin (SEPAA 0.5 and SEPAA 1.0, respectively; Scheme 1, Structure 1) were prepared and identified by the methods described elsewhere [13, 14]. The synthesis and identification of sulfoethylated polyethyleneimines with a DM of 0.30, 0.58, and 0.74 crosslinked with diethylene glycol diglycidyl ether (SEPEI 0.30, SEPEI 0.58 and SEPEI 0.74, respectively; Scheme 1, structure 2) are presented in [14]. Sulfoethylated chitosans with a DM of 0.3, 0.5, 0.7, and 1.0 crosslinked with glutaraldehyde (SEC 0.3, SEC 0.5, SEC 0.7 and SEC 1.0, respectively; Scheme 1, structure 3) were prepared and identified in accordance with the procedures described in [12].
The degrees of sulfoethyl modification (DM) of aminopolymers were calculated as DM = nS/nN, where nS and nN, respectively, are the amounts of sulfur and nitrogen as probed by elemental analysis.

Scheme 1 . The structures of taurine-derived sorption materials, where DM is the degree of modification of polymer amino groups by sulfoethyl groups
Kinetic Studies of Metal Ion Sorption on Sulfoethylated Aminopolymers
0.1-mol/L stock solutions of copper(II), nickel(II), cobalt(II), zinc(II), cadmium(II), calcium(II), magnesium(II), strontium(II), barium(II), and silver(I) nitrates were prepared from the corresponding metal nitrates of pure for analysis grade (purchased from Vekton). Solutions with lower concentrations were prepared by successively diluting aliquots of the standard solution with deionized water.
The sorption kinetics of silver(I), copper(II), nickel(II), cobalt(II), zinc(II), cadmium(II), calcium(II), magnesium(II), strontium(II), and barium(II) ions on sulfoethylated aminopolymer sorbents were studied under batch conditions with constant stirring using an Ekros 6410 M stirrer. In order to assess an option to vary the selectivity of metal ion sorption from multicomponent solutions depending on the phase contact time, we carried out an experiment where all metal ions were simultaneously present in the solution. The initial concentration of metal ions in the solution was 1 × 10–4 mol/L. The pH was maintained constant by means of an ammonium acetate buffer solution. The sorption kinetics of metal ions on SEC were studied at рН 6.5, on SEPAA at рН 5.0, and on SEPEI at рН 4.0 and 7.0. The initial concentrations of acetic acid and ammonia solutions were 0.34 and 0.37 mol/L, respectively. The pH was adjusted to a tailored value by gradual dropwise addition of aqueous ammonia. The solution acidity was monitored on an I-160 MI ion meter equipped with an ESK-10601/7 combination glass electrode. Deionized water was produced using the Millipore Milli-Q Academic High Water Purification System. The test solution volume was 50.0 mL; the sorbent weight was 0.0200 g. Once the prescribed contact time between the sorbent and solution elapsed, the phases were separated using a “Blue Ribbon” filter. The metal ions in solutions before and after sorption were determined by inductively coupled plasma atomic emission spectroscopy (iCAP 6500 “ThermoElectron”). The difference between the concentrations of solutions before and after interaction with the sorbent served to calculate the recovery of metal ions (a, mmol/g) and to plot kinetic curves in the space a = f(t), where t is the phase contact time. The distribution ratios of metal ions (D) between the solution and sorbent were calculated as the ratio of the metal ion concentration in the sorbent phase to the ion concentration in the solution.
RESULTS AND DISCUSSION
Metal Ion Sorption Kinetics on Sulfoethylated Aminopolymers
The sorption kinetics of metal ions on polyallylamine sorbents with various DMs was studied at pH 5.0, which value corresponds to the highest selectivity of silver(I) sorption compared to that of copper(II) sorption [13]. As an example, Fig. 1 shows the rate curves obtained for SEPAA 0.5. SEPAA 1.0 has similar metal ion sorption rate curves.
SEPAAs selectively recover silver(I) ions over the entire range of phase contact times studied. The other metal ions are only marginally extracted by SEPAAs. Silver(I) ion sorption equilibrium is attained in 30 min of phase contact.
In order to determine the stage that controls the overall rate of the sorption process, we carried out mathematical processing of the obtained dependences using diffusion and chemical kinetic models. At the first stage, where rate curves were plotted in the spaces ln(1 – F)–τ and aτ–τ1/2 (τ is time, F is degree of equilibration of the system calculated as F = aτ/ae, where aτ is the amount of the material sorbed at moment τ, mmol/g; and ae is the amount of the material sorbed under equilibrium, mmol/g), it was found that external and internal diffusion models [15, 16], respectively, poorly describe the experimental data set for silver(I) ion sorption on SEPAAs.
We used pseudo-first-order and pseudo-second-order models [17, 18] and Elovich’s model [19, 20] as the chemical kinetic models to process silver(I) sorption curves on SEPAAs. The mathematical processing of silver(I) sorption curves on SEPAAs with various DMs gave the following parameters: ae, the amount of sorbate metal per unit weight of sorbent in equilibrium, mmol/g; k1, the sorption rate constant of the pseudo-first-order model, min–1; k2, the sorption rate constant of the pseudo-second-order model, g/(mmol min); α, the initial sorption rate, g/(mmol min); β, the constant of Elovich’s equation corresponding to the surface coverage of the sorbent and the activation energy of chemisorption, g/mmol; and determination coefficients R2 (Table 1).
The values of determination coefficients (Table 1) imply that the pseudo-first-order model best of all fits silver(I) sorption on SEPAAs. Therefore, the rate-controlling step is the interaction of silver(I) with sorbent functions.
It was found that k1, the rate constant of silver(I) sorption, decreases as the sorbent DM rises. A similar trend is typical of k2, the parameter of the pseudo-second-order model for which high determination coefficients are also obtained. The equilibrium silver(I) recovery on SEPAA increases in association.
Metal Ion Sorption Kinetics on Sulfoethylated Chitosans
The silver(I) sorption kinetics from multicomponent solutions was also studied at рН 6.5 for SEC materials with various DMs: 0.3, 0.5, 0.7, and 1.0. As an example, Fig. 2 shows the metal ion sorption rate curves for a sorbent with an average degree of modification, namely, for SEC 0.5. Unlike SEPAA, SEC recovers copper(II) ions from multicomponent solutions along with silver(I). The increasing sorbent DM only insignificantly changes silver(I) recovery and decreases copper(II) recovery. Accordingly, the silver(I) sorption selectivity rises relative to copper(II).
Integral metal ion sorption rate curves on SEC 0.5 in case where the metal ions are simultaneously present in ammonium acetate buffer solution, рН 6.5. The initial metal ion concentration: 1 × 10–4 mol/L. (1) Ag(I), (2) Cu(II), and (3–10) Ni(II), Ba(II), Sr(II), Cd(II), Ca(II), Mg(II), Zn(II), and Co(II).
All studied sorbents recovered copper(II) and silver(I) ions to a large extent as rapidly as in the first 30 min of phase contact. For SEC 0.5 and SEC 0.7, no further increase in recovery was observed with a longer phase contact time. For SEC 0.3 and SEC 1.0, after a sharp increase in the first moments of contact between phases, the copper(II) and silver(I) recovery slowly increases to reach a peak value in 24 h. This circumstance is explained by the crosslinking specifics of the sorbents under consideration. Since glutaraldehyde is present in aqueous solutions in various species [21, 22], its interaction with SEC forms a specific set of nitrogen-containing functional groups.
We used the parameters determined in the processing of the rate curves (Table 2) to calculate the initial silver(I) sorption rates (mmol/(min g)) on the sorbents studied in accordance with the expression taken from [23]:
Figure 3 shows the initial silver(I) sorption rates on sulfoethylated aminopolymer sorbents as a function of DM. Our prior results, in particular, [24], were used to calculate the initial silver(I) sorption rates on sulfoethylated polyaminostyrenes (SEPAS; Scheme 1, structure 4).
The highest silver(I) sorption rates were found for SEC 0.5 and SEC 0.7, which is consistent with their shorter equilibration times compared to SEC 0.3 and SEC 1.0. In general, however, SECs and SEPAAs with various DMs have similar values of the initial silver(I) sorption rates. The copper(II) sorption rates on SECs with various DMs fall in the range 3.2 × 10–4–5.5 × 10–4 mmol/(min g). The far lower values of this parameter for copper(II) compared to those for silver(I) are a favorable factor in terms of achieving high sorption selectivity of the latter under dynamic conditions, where the efficiency of metal ion separation is largely determined by the process kinetics.
In most cases, the pseudo-second-order and Elovich’s models offer the best fit to the silver(I) and copper(II) sorption rate curves on SEC sorbents. Elovich’s model best fits the curves for SEC 0.3 and SEC 1.0, indicating a high energy inhomogeneity of the sorbent surface according to the data of [19, 20].
Metal Ion Sorption Kinetics on Sulfoethylated Polyethyleneimines
Previously [14], we found that polyethyleneimine-based sorbents (SEPEIs), unlike other sulfoethylated aminopolymers, can act, depending on the acidity of the medium, both as group sorbents for the extraction of a number of transition-metal ions and for the selective recovery of copper(II) and silver(I). For this reason, the metal ion sorption rate curves for SEPEIs were measured at the corresponding pH values.
At pH 4.0, the sorbents selectively recover copper(II) and silver(I) ions over the entire range of phase contact times. The recovery of the studied metal ions decreases as the DM of SEPEI rises. This is due to the basicity of amino groups in the sorbent decreasing due to an increase in the content of sulfoethyl groups, which have a negative inductive effect. The stability of the complexes formed by metal ions in the SEPEI phases decreases, too.
The metal ion sorption rate curves on SEPEIs with various DMs at рН 7.0 are shown in Fig. 4. It is natural that under the experimental conditions where the degree of deprotonation of the SEPEI amino groups is higher than in sorption from more acidic media, it acts as a group sorbent with respect to a number of metal ions. So, SEPEI 0.34 at pH 7.0 co-recovers silver(I), copper(II), nickel(II), cobalt(II), cadmium(II), and zinc(II), while SEPEI 0.74 co-recovers silver(I), copper(II), and nickel(II); that is, a rise in DM of SEPEI at рН 7.0 increases the sorption selectivity for some metal ions, precisely as at рН 4.0.
Integral metal ion sorption rate curves on SEPEI with (a) DM of 0.34 and (b) DM of 0.74 in case where the metal ions are simultaneously present in ammonium acetate buffer solution, рН 7.0. The initial metal ion concentration: 1 × 10–4 mol/L, g(sorbent) = 0.0200 g. Metal ions: (а) (1) Ni(II), (2) Cu(II), (3) Ag(I), (4) Cd(II), (5) Co(II); (6) Zn(II), (7–10) Ca(II), Mg(II), Ba(II), and Sr(II); (b) (1) Ag(I), (2) Cu(II), (3) Ni(II), (4) Cd(II), (5) Co(II), and (6–10) Zn(II), Ca(II), Mg(II), Ba(II), and Sr(II).
In both cases (at pH 4.0 and 7.0), an increase in DM of SEPEI decreases the equilibrium acquisition time for the studied (metal salt solution)–SEPEI systems. At рН 4.0, for example, the equilibrium acquisition time decreases from 120 to 40 min as the sorbent DM rises from 0.30 to 0.74. At рН 7.0, the equilibrium acquisition time is 400 min for SEPEI 0.34 and 60 min for SEPEI 0.74.
The obtained data were used to calculate silver(I) distribution ratios (Table 3).
In order to identify the stage that controls the overall rate of the process, we mathematically processed the integral sorption rate curves by diffusion and chemical kinetic equations. Just as for SEPAA and SEC, chemical kinetic models in general better fit the experimental data. The respective correlation coefficients appear in Tables 4 and 5. The parameters obtained in processing the metal ion sorption rate curves on SEPEI are listed in Tables 4 and 6.
From Fig. 3, one can infer that SEPEI has initial sorption rates comparable to those on SEC and SEPAA. Intriguingly, the respective values for silver(I) and copper(II) have antibate trends. At рН 4.0, for example, the silver(I) sorption rate decreases in response to rising DM of SEPEI, while the copper(II) sorption rate increases from 0.04 to 0.17 mmol/(min g). At рН 7.0, on the contrary, the silver(I) sorption rate on SEPEI increases, while the copper(II) sorption rate decreases from 0.06 to 0.04 mmol/(min g). This circumstance can be explained both by the mutual influence of metal ions during their SEPEI sorption, and by the different polymer chain conformations at different acidities.
Summing up our study of the sorption kinetics of metal ions on taurine derivative sorbents, we can draw several conclusions. For SEC, SEPAA, and SEPEI sorbents, the chemical structure of the polymer matrix and the degree of modification by sulfoethyl groups do not have a significant effect on the initial silver(I) sorption rate. Slightly differing trends are typical of polyaminostyrene (SEPAS) sorbents, the properties of which have been studied earlier [24]. SEPAS sorbents are distinguished by far higher initial sorption rates (Fig. 3) and, accordingly, the least sorption equilibrium acquisition times. This circumstance is due to the specifics of the supramolecular structure of SEPAS. Additional crosslinking of the polymer was not carried out during the synthesis of this material, as the network structure of the polymer was formed upon the reduction of nitropolystyrene due to the formation of imino groups [24]. To prepare water-insoluble SEC, SEPAA, and SEPEI sorbents, crosslinking agents were used, which was responsible for a higher degree of crosslinking of these materials. The specifics of the sorption behavior of SEPAS demonstrate that the supramolecular structure is the main factor affecting the kinetic characteristics of the sorbents produced by polymer-analogous transformations. Intentionally forming the supramolecular structure during crosslinking of linear polymers via selecting the crosslinking agent and crosslinking conditions, one has a tool to set the necessary and required performances of the final sorption material.
CONCLUSIONS
Sorption rate curves have been obtained for the recovery of some metal ions from multicomponent solutions on materials with taurine functions depending on the degree of modification of the material by sulfoethyl groups and the nature of the aminopolymer matrix. Polyallylamine-based materials selectively recover silver(I) ions from multicomponent solutions over the entire range of phase contact times, while chitosan-based materials do the same for copper(II) and silver(I) ions. Sulfoethylated polyethyleneimine materials can be used, depending on the solution acidity, as sorbents for the group recovery of transition-metal ions or for co-recovering copper(II) and silver(I). The initial silver(I) and copper(II) sorption rates on sulfoethylated aminopolymers have been calculated for the studied (metal salt solution)–sorbent systems. The degree of modification and the nature of the polymer matrix do not affect significantly the kinetic parameters of silver(I) sorption in case of SEC, SEPEI, and SEPAA. SEPAS sorbents show the highest sorption rates due to the specifics of their supramolecular structures. In addition, SEPAS and SEPAA both have high silver(I) sorption selectivities. In general, all the materials under study recover sorbable metal ions to a large extent as early as in the first minutes of phase contact, which determines their prospects for use in separation and concentration processes.
REFERENCES
C. Ghiorghita, K. Borchert, A. Vasiliu, et al., Colloids Surf., A: Physicochem. 607, 125504 (2020). https://doi.org/10.1016/j.colsurfa.2020.125504
A. V. Pestov, Yu. O. Privar, A. V. Mekhaev, et al., Eur. Polym. J. 115, 356 (2019). https://doi.org/10.1016/j.eurpolymj.2019.03.049
L. K. Neudachina, Yu. S. Petrova, A. S. Zasukhin, et al., Anal. Kontrol 15, 88 (2011).
R. Kh. Khamizov, Russ. J. Phys. Chem. 94, 171 (2020). https://doi.org/10.1134/S0036024420010148
R. Kh. Khamizov, D. A. Sveshnikova, A. E. Kucherova, et al., Russ. J. Phys. Chem. 92, 1782 (2018). https://doi.org/10.1134/S0036024418090121
M. V. Maslova, V. I. Ivanenko, and L. G. Gerasimova, Russ. J. Phys. Chem. 93, 1245 (2019). https://doi.org/10.1134/S0036024419060219
N. S. Jalbani, A. R. Solangi, S. Memon, et al., J. Mol. Liq. 339, 116741 (2021). https://doi.org/10.1016/j.molliq.2021.116741
T. Manobala, S. Shukla, T. S. Rao, et al., Chemosphere 269, 128722 (2021). https://doi.org/10.1016/j.chemosphere.2020.128722
S. B. Yarusova, N. V. Makarenko, P. S. Gordienko, et al., Russ. J. Phys. Chem. 92, 559 (2018). https://doi.org/10.1134/S0036024418030354
A. V. Dolganov, A. V. Balandina, D. B. Chugunov, et al., Russ. J. Inorg. Chem. 65, 1770 (2020). https://doi.org/10.1134/S0036023620110030
G. Duca, I. Zinicovscaia, and D. Grozdov, Russ. J. Gen. Chem. 90, 2546 (2020). https://doi.org/10.1134/S1070363220130034
Yu. S. Petrova, A. V. Pestov, M. K. Usoltseva, et al., J. Hazard. Mater 299, 696 (2015). https://doi.org/10.1016/j.jhazmat.2015.08.001
L. M. K. Alifkhanova, K. Y. Lopunova, A. V. Pestov, et al., Sep. Sci. Technol. 56, 1303 (2021). https://doi.org/10.1080/01496395.2020.1781175
E. I. Kapitanova, E. O. Zemlyakova, A. V. Pestov, et al., Russ. Chem. Bull. 68, 1252 (2019). https://doi.org/10.1007/s11172-019-2549-5
G. E. Boyd, A. W. Adamson, and L. S. Myers, J. Am. Chem. Soc. 69, 2836 (1947). https://doi.org/10.1021/ja01203a066
Sh. Sharma and N. Rajesh, J. Environ. Chem. Eng. 4, 4287 (2016). https://doi.org/10.1016/j.jece.2016.09.028
Y. S. Ho and G. McKay, Process Biochem. 34, 451 (1999). https://doi.org/10.1016/S0032-9592(98)00112-5
T. L. Lin and H. L. Lien, Int. J. Mol. Sci. 14, 9834 (2013). https://doi.org/10.3390/ijms14059834
S. Y. Elovich and O. G. Larinov, Izv. Akad. Nauk SSSR 2, 209 (1962).
R. Han, W. Zou, Z. Zhang, et al., J. Hazard. Mater. 137, 384 (2006). https://doi.org/10.1016/j.jhazmat.2006.02.021
P. A. Perminov, N. R. Kil’deeva, L. M. Timofeeva, et al., Izv. Vyssh. Ucheb. Zav., Ser. Khim. Khim. Tekhnol. 50, 53 (2007).
N. R. Kildeeva, P. A. Perminov, L. V. Vladimirov, et al., Russ. J. Bioorg. Chem. 35, 360 (2009).
M. Ozacar, Process Biochem. 40, 565 (2005). https://doi.org/10.1016/j.procbio.2004.01.032
L. M. k. Alifkhanova, O. I. Merezhnikova, Yu. S. Petrova, et al., Russ. J. Appl. Chem. 93, 1392 (2020). https://doi.org/10.1134/S1070427220090128
Funding
The work was supported by the Russian Science Foundation (project No. 21-73-00052), https://rscf.ru/project/21-73-00052.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Petrova, Y.S., Alifkhanova, L.M., Kuznetsova, K.Y. et al. Sorbents with a Taurine Function: Kinetics of Interaction with Singly and Doubly Charged Metal Ions in Ammonium Acetate Buffer Solution. Russ. J. Inorg. Chem. 67, 1080–1087 (2022). https://doi.org/10.1134/S003602362207018X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362207018X