Abstract
Materials based on graphitic carbon nitride (g-C3N4) are intensely studied as promising photocatalysts of different reactions, hydrogen peroxide formation including. Effect of synthesis parameters of g-C3N4 obtained by thermolysis of melamine, urea, and thiourea on its composition and photocatalytic activity has been studied. The photocatalytic activity of obtained materials has been studied in the reaction of oxidative decomposition of organic dye and in oxygen reduction to form hydrogen peroxide. It has been shown that, in spite of incomplete polycondensation after thermolysis, the obtained samples display high values of photocatalytic activity. The work has shown that the photocatalytic activity of samples obtained at 550°С is by factor 2–4 higher than that for samples obtained at 500 and 600°C. It has been shown that the preparation of g-C3N4 from thiourea in air leads to photocatalyst with maximal activity, whereas melamine and urea produce the best catalyst under nitrogen atmosphere.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Graphitic carbon nitride (g-C3N4) is a promising semiconducting material, which is intensely studied in recent time and can be used as efficient photocatalyst [1–5], electrocatalyst [6–8], material for light-emitting devices [9] and supercapacitors [10]. g-C3N4 meets many requirements for photocatalysts, namely: it is thermally and chemically stable [11, 12], has appropriate valence band and conduction band valley [13, 14], can be obtained from available compounds such as melamine, urea, thiourea, dicyandiamide [15, 16], and has small band gap that provides visible light absorption. Due to these properties, graphitic carbon nitride is considered as promising photocatalyst for water decomposition to form gaseous oxygen and hydrogen, CO2 reduction, and oxidation of different organic contaminants.
Hydrogen peroxide generation is one of promising and intensely studied in recent time photocatalytic processes [17–20]. Hydrogen peroxide can be safely stored; it is an excellent environmentally benign oxidizing agent and is used in organic synthesis, biomedicine, and biosensorics [21–25]. Because of the absence of gaseous oxygen–hydrogen mixture on synthesis, the photocatalytic method of hydrogen peroxide production is potentially more advantageous and environmentally safe. Since photocatalytic formation of hydrogen peroxide is caused by oxygen reduction, required photocatalyst should have high reductive potential of photogenerated electrons. Graphitic carbon nitride may suit these requirements due to high position of conduction band and small energy gap width. In spite of high activity of graphitic carbon nitride materials in photocatalytic synthesis of hydrogen peroxide, the majority of works is devoted to photocatalysis on composite materials, whereas the effect of parameters of g-C3N4 synthesis on its properties is studied insufficiently.
The study of formation of graphitic carbon nitride showed that the polycondensation of urea and thiourea leads to production of g-C3N4 through formation of melamine followed by emergence of heptazine units and their subsequent coupling [15]. The composition and structure of final carbon nitride depends on completeness of heptazine units polycondensation, which is determined by synthesis conditions such as annealing temperature, duration, and atmosphere.
In the present work, we studied effect of certain parameters of synthesis of graphitic carbon nitride on the activity of resultant photocatalyst in the production of hydrogen peroxide. Samples were obtained by thermolysis of urea, melamine, and thiourea in air and under nitrogen atmosphere in the temperature range of 500–600°C. Since the temperature range of g‑C3N4 formation is close to its decomposition temperature (~650°C), special attention in the work was paid to assessment of yield of sample synthesis reactions, completeness of graphitic structure formation and concentration of functional groups on the surface. The obtained materials were studied in terms of composition and structure, while their photocatalytic activity was measured in both reactions of decomposition of model organic dye and the reactions of photocatalytic production of hydrogen peroxide in aqueous alcoholic solution.
EXPERIMENTAL
The samples of graphitic carbon nitride were obtained by the thermal decomposition of organic precursors with high nitrogen content (melamine, urea, and thiourea). A 5-g weighed sample of precursor was placed in a closed porcelain crucible and annealed at 500–600°C for 1–4 h in a muffle furnace in air or in a tube furnace in nitrogen flow. Heating rate in all experiments was 5 K/min. The obtained samples containing g-C3N4 were studied without further purification.
X-ray powder diffraction study was performed on a Rigaku D/MAX 2500 diffractometer with rotating anode (Japan) in reflection mode (Bragg–Brentano geometry) using CuKα1,2 radiation and graphite monochromator.
X-ray photoelectron spectra were registered on an ESCA facility of NanoPES Endstation at the Kurchatov synchrotron radiation source (Kurchatov Research Center) equipped with a SPECS Phoibos 150 high-resolution electron energy hemispherical analyzer with monochromatic X-ray radiation source (AlKα, excitation energy of 1486.61 eV, ∆E = 0.2 eV) [26]. Studied powder samples were pressed into indium foil and attached to a manipulator.
IR spectra were recorded on a Bruker ALPHA Fourier-transform IR spectrometer (Germany) in the range of 400–4000 cm–1 in frustrated total internal reflection mode. The content of carbon, hydrogen, and nitrogen was measured with a Carlo Erba Instruments EA 1108 CHN analyzer (Italy).
Specific surface area was measured by low-temperature nitrogen sorption at T = 77 K on a Quantachrome NOVA 4200e instrument (USA). Samples were preliminary degassed at 200°С in vacuum for 2 h. Obtained adsorption–desorption isotherms were used to determine specific surface area of samples by Brunauer–Emmett–Teller model.
To study kinetics of photocatalytic hydrogen peroxide formation, 0.2 mL of ethanol and 0.3 mL of 0.1 M phosphate buffer solution was added to 2.5 mL of aqueous suspension of photocatalyst with concentration of 0.1 mg/mL. The suspension was exposed to irradiation of UV lamp (wavelength 366 nm) of CAMAG TLC Visualizer 2 instrument (Switzerland) with stirring. The concentration of evolved hydrogen peroxide was determined by the specific enzymatic reaction of 3,3',5,5'-tetramethylbenzidine (TMB) oxidation by horseradish peroxidase; for this purpose, 60-µL aliquots were taken each 5 min and added in a 96-well plate with a mixture of 204 µL of 0.1 M phosphate buffer, 6 µL of 5 × 10–5 M solution of horseradish peroxidase, and 30 µL of 10–3 M alcoholic TMB solution. The concentration of oxidized TMB was determined by spectrophotometry using a BMG Labtech SPECTROstar Nano spectrometer (Germany) with accuracy of ± 0.003 optical units.
The photocatalytic properties of obtained samples in the reaction of decomposition of methylene blue model dye were measured using previously described flow-type measurement installation [27, 28]. A dispersed weighed sample of photocatalyst was exposed to UV radiation using a 5.5-W high-pressure cylindrical mercury lamp immersed in reaction mixture. methylene blue was used as a model dye at concentration of 25 mg/L. Continuous sampling was performed during experiment using a peristaltic pump, solution was passed through a U-shaped cuvette where optical absorption spectrum was measured each 3 s. The spectra were measured using a 500-W Ocean Optics HRX-2000 xenon lamp (USA) and an Ocean Optics QE65000 spectrophotometer.
RESULTS AND DISCUSSION
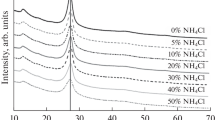
g-C3N4 samples were obtained in both air and under nitrogen atmosphere at 500–600°C. Increase in annealing duration and temperature led to decrease of yield for all precursors. The use of urea as precursor during annealing for 4 h at 600°C led to its complete decomposition in air, whereas thiourea under these conditions completely degraded in nitrogen. All obtained samples represent monophase carbon nitride with heptazine structure, typical XRD patterns of g-C3N4 are shown in Fig. 1a. X-ray powder diffraction (XRD) analysis for samples obtained from melamine displays peaks with smaller half-width probably due to increase in coherent-scattering regions and decrease of the number of defects in structure. When urea and thiourea were used as precursors, reflections on XRD pattern are considerably broadened, which indicates low structure ordering of obtained samples.
The IR spectra of obtained samples also confirm the formation of graphitic carbon nitride (Fig. 1b). The spectra of all samples exhibit vibrations of C=N bonds (1625 cm–1), vibrations corresponding to heptazine (883 cm–1) and triazine fragments (800 cm–1), vibrations of aromatic rings (1550–1300 cm–1) and residues of non-condensed intermediate products containing NH and NH2 groups (3300–3050 cm–1). The presence of amino groups indicates that polycondensation process is incomplete.
The results of CHN analysis (Table 1) indicate that obtained samples contain hydrogen, while the share of nitrogen is higher as compared with the nominal composition of g-C3N4 (N/C = 1.33) and lays in the range 1.35–1.46. These N/C values and the results of IR spectroscopy indicate the presence of non-condensed fragments in the structure of g-C3N4 on thermolysis of organic precursors. Nonetheless, the composition is close to C6N9H4 (N/C = 1.5) in the case of polycondensed one-dimensional chain of heptazine units, which is higher that the obtained by us values and indicates the presence of two-dimensional layers of carbon nitride with heptazine structure.
The incomplete joining of polycondensed heptazine chains into two-dimensional layers is also confirmed by X-ray photoelectron spectra (XPS) (Fig. 2, Table 2). So, XPS in the region of binding energy of C1s line shows the main peak corresponding to carbon in g‑C3N4 structure, namely N–C=N (289.3 eV) and the peaks of additional admixtures on the surface: С–С (285.6 eV), С–OH (287.2 eV), and O–C=O (291 eV). XPS in the region of binding energy of N1s line displays the peaks corresponding to nitrogen atoms in graphitic carbon nitride: C–N=C (399.7 eV) and N–(C3) (400.7 eV), as well as NHx (401–402 eV) and N–O (402–403 eV) corresponding to nitrogen in admixtures and functional groups of the surface. Thus, taking into account the results of CHN analysis, IR spectroscopy, and XPS, one can conclude that the obtained samples contain a considerable fraction of incompletely condensed heptazine layers.
The photocatalytic activity of obtained g-C3N4 samples was studied in the reaction of decomposition of methylene blue model dye and the photocatalytic reduction of oxygen dissolved in water–alcohol mixture into hydrogen peroxide. Since the positions of valence band and conduction band of carbon nitride have more negative potential as compared with oxide semiconductors, photoelectrons can participate in reduction reactions.
Figure 3 displays the values of reaction rate constants for photocatalytic formation of hydrogen peroxide in the presence of catalysts obtained by annealing in air and nitrogen for 4 h. In the case of g-C3N4 samples obtained by urea annealing, the large values of reaction rate constants are observed in the synthesis under nitrogen atmosphere. This fact may be caused by the lower stability of the obtained samples toward oxidation and further decomposition during synthesis, which is also confirmed by the low yields of g-C3N4 formation from urea in air as compared with nitrogen atmosphere. The samples obtained from thiourea, on the contrary, show higher activity after synthesis under nitrogen atmosphere. The comparison of photoelectron spectra showed that g-C3N4 samples obtained from thiourea under nitrogen atmosphere contain much larger amino groups, which indicates lower polycondensation as compared with samples obtained from thiourea in air.
g-C3N4 samples obtained from melamine display lower values of H2O2 formation constants as compared with samples obtained from other precursors. In spite of higher crystallinity of samples obtained from melamine according to XRD, they have relatively low activity, which can be explained by lower specific surface area. So, according to the data of low-temperature nitrogen adsorption, a g-C3N4 sample obtained by melamine thermolysis at 550°C for 4 h under nitrogen atmosphere has specific surface area of ~8 m2/g, whereas g-C3N4 samples obtained under similar conditions from urea and thiourea display these values of 26 and 23 m2/g, respectively.
The study of photocatalytic activity of obtained g-C3N4 samples in the reaction of decomposition of methylene blue model dye (Fig. 4) showed that samples obtained in nitrogen have higher values of decomposition constant.
CONCLUSIONS
We have studied the effect of parameters of graphitic carbon nitride synthesis from different precursors on its photocatalytic activity. The used synthesis conditions provide no complete polycondensation of graphitic structure: all obtained samples contain considerable fraction of amino groups on the surface. Nonetheless, the obtained samples display high photocatalytic activity in the reactions of hydrogen peroxide formation (up to 1 mM/min) and methylene blue decomposition (1.8 × 10–1 g–1 s–1), while the key parameters determining photocatalytic activity of g-C3N4 are temperature and atmosphere of synthesis. In the case of samples obtained from melamine and urea, products obtained under nitrogen atmosphere display higher activity, whereas the highest activity is observed for products obtained in air from thiourea as a precursor.
REFERENCES
Y. M. Zhang, C. W. An, D. F. Zhang, et al., Russ. J. Inorg. Chem. 66, 679 (2021). https://doi.org/10.1134/S0036023621050223
J. Zhu, P. Xiao, H. Li, et al., ACS Appl. Mater. Interfaces 6, 16449 (2014). https://doi.org/10.1021/am502925j
S. Cao, J. Low, J. Yu, et al., Adv. Mater. 27, 2150 (2015). https://doi.org/10.1002/adma.201500033
X. Wang, S. Blechert, and M. Antonietti, ACS Catal. 2, 1596 (2012). https://doi.org/10.1021/cs300240x
M. I. Chebanenko, N. V. Zakharova, and V. I. Popkov, Russ. J. Appl. Chem. 93, 494 (2020). https://doi.org/10.1134/S1070427220040035
M. I. Chebanenko, N. V. Zakharova, A. A. Lobinsky, et al., Semiconductors 53, 2072 (2019). https://doi.org/10.1134/S106378261912008X
L. Wang and W. A. Daoud, Appl. Surf. Sci. 324, 532 (2015). https://doi.org/10.1016/j.apsusc.2014.10.11
S. S. Shinde, A. Sami, and J.-H. Lee, ChemCatChem 7, 3873 (2015). https://doi.org/10.1002/cctc.201500701
L. He, M. Fei, J. Chen, et al., Mater. Today (2019). https://doi.org/10.1016/j.mattod.2018.06.008
S.-X. Zhou, X.-Y. Tao, J. Ma, et al., Vacuum 149, 175 (2018). https://doi.org/10.1016/j.vacuum.2017.12.019
S. C. Yan, Z. S. Li, and Z. G. Zou, Langmuir 25, 10397 (2009). https://doi.org/10.1021/la900923z
E. G. Gillan, Chem. Mater. 12, 3906 (2000). https://doi.org/10.1021/cm000570y
Y. Zheng, L. Lin, B. Wang, et al., Angew. Chem., Int. Ed. Engl. 54, 12868 (2015). https://doi.org/10.1002/anie.201501788
X. Li, J. Yu, J. Low, et al., J. Mater. Chem. A 3, 2485 (2015). https://doi.org/10.1039/C4TA04461D
I. Papailias, T. Giannakopoulou, N. Todorova, et al., Appl. Surf. Sci. 358, 278 (2015). https://doi.org/10.1016/j.apsusc.2015.08.097
Z. Zhao, Y. Sun, Q. Luo, et al., Sci. Rep. 5, 14643 (2015). https://doi.org/10.1038/srep14643
H. Hou, X. Zeng, and X. Zhang, Angew. Chem., Int. Ed. Engl. 59, 17356 (2020). https://doi.org/10.1002/anie.201911609
C. Chu, D. Huang, Q. Zhu, et al., ACS Catal. 9, 626 (2019). https://doi.org/10.1021/acscatal.8b03738
Y. Shiraishi, S. Kanazawa, Y. Kofuji, et al., Angew. Chem., Int. Ed. Engl. 53, 13454 (2014). https://doi.org/10.1002/anie.201407938
K. Sahel, L. Elsellami, I. Mirali, et al., Appl. Catal., B 188, 106 (2016). https://doi.org/10.1016/j.apcatb.2015.12.044
H. Dong, X. Guan, D. Wang, et al., Chemosphere 85, 1115 (2011). https://doi.org/10.1016/j.chemosphere.2011.07.029
R. Baciocchi, M. R. Boni, and L. D’Aprile, J. Hazard. Mater. 107, 97 (2004). https://doi.org/10.1016/j.jhazmat.2003.09.008
K. Jóźwiakowski, M. Marzec, J. Fiedurek, et al., Sep. Purif. Technol. 173, 357 (2017). https://doi.org/10.1016/j.seppur.2016.08.047
T. Wang, L. Yang, B. Zhang, et al., Colloids Surf., B: Biointerfaces 80, 94 (2010). https://doi.org/10.1016/j.colsurfb.2010.05.041
O. R. Simonova, S. A. Zdanovich, S. V. Zaitseva, et al., Russ. J. Inorg. Chem. 65, 1006 (2020). https://doi.org/10.1134/S0036023620070207
A. M. Lebedev, K. A. Menshikov, V. G. Nazin, et al., J. Surf. Invest. X-ray, Synchrotron Neutron Tech. 15, 1039. https://doi.org/10.1134/S1027451021050335
V. A. Lebedev, V. V. Sudin, D. A. Kozlov, et al., Nanotechnologies Russ. 11, 20 (2016). https://doi.org/10.1134/S1995078016010092
D. A. Kozlov, A. B. Shcherbakov, T. O. Kozlova, et al., Molecules 25 (1), 154 (2019). https://doi.org/10.3390/molecules25010154
Funding
This work was financially supported by the Russian Foundation for Basic Research (project no. 20-33-90333). The study was carried out using equipment of the Shared Facility Center, Moscow State University, “Technologies of production of nanostructurized materials and their integrated study” purchased by the Moscow State University in the context of the Nauka National Project and the MSU Development Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Kozlov, D.A., Artamonov, K.A., Revenko, A.O. et al. Effect of the Graphitic Carbon Nitride Synthesis Atmosphere on its Activity in the Photocatalytic Generation of Hydrogen Peroxide. Russ. J. Inorg. Chem. 67, 715–720 (2022). https://doi.org/10.1134/S0036023622050102
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050102