Abstract
Activity coefficients in aqueous solutions of alkali metal nitrates at 298 K have been calculated by the generalized Debye–Huckel theory using experimental values of the static dielectric constant of solutions. It has been shown that calculation without optimization of model parameters in a number of systems reproduces the dependence of activity coefficients on cation radius and the levelling of potassium, rubidium, and cesium cations influence on activity coefficients in the presence of nitrate ions. The effect of hydration and ion association on the thermodynamic properties in the series of studied systems has been considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In recent years, the generalized Debye–Hückel theory (GDHT) developed by us in 2015 [1] is used for the analysis of interrelation for dielectric, thermodynamic, and structural properties of electrolyte solutions. Considered systems included aqueous solutions of chlorides [1], iodides [2], and sulfates [3] of alkali metals, sodium [4] and aluminum [5] salts with different anions. The use of GDHT allows computation of activity coefficients without optimization of model parameters employing experimental values of static dielectric constant (DC) for solutions, which is determined by dielectric spectroscopy methods [6, 7]. Therefore, the description of activity coefficients of water and ions accentuates the detection and analysis of different physicochemical factors and phenomena affecting the thermodynamic and dielectric properties of solutions rather than quantitative coincidence with experimental data. To extend the range of systems studied by this method, we devoted this paper to the consideration of interrelation of DC and activity coefficients in aqueous solutions of alkali metal nitrates at 298 K.
CONCENTRATION DEPENDENCE OF DIELECTRIC CONSTANT
The dielectric properties of solutions of alkali metal nitrates were studied in the works [8–12]. Data on static DC that cover all five systems were used for calculations [8, 9]. The complex DC ε*(ω) of solutions in these works was determined in frequency range Δν = 2.4–12 GHz and approximated by Debye equation to determine static DC by extrapolation to zero frequency. In the later works [10–12], complex DC was measured in a wider frequency range, while extrapolation employed more complicated model, Cole–Cole equation. The calculated values of static DC in these works proved to be higher than the data of works [8, 9]. However, we do not use these results in the present work because they are incomplete.
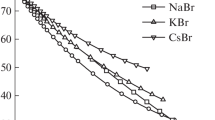
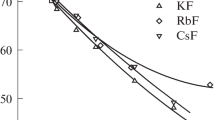
For the use in the calculation of activity coefficients, we approximated solution DC by analytical expressions presented in Table 1. For DC of pure water, we used the value from [13]. As seen from Table 1 and Fig. 1, DC increases with cation radius at the constant solution concentration, which reflects the weakening of its hydration. The same feature is observed for a number of alkali metal chlorides and iodides [1, 2].
CALCULATION OF ACTIVITY COEFFICIENTS AND RESULTS AND DISCUSSION
Activity coefficients for the components of studied solutions were calculated using GDHT equations, which were discussed in detail in previous publications [1, 2, 4]. Remember that activity coefficient logarithms in this model are calculated as the sum of contribution of Coulomb ion–ion interactions and solvation contribution. The first contribution is similar to that considered in common Debye–Hückel theory (with allowance made for ion size), while the second contribution characterizes the interaction of ions with environment containing both solvent and ions. Both contributions allow for the concentration dependence of solution DC.
Model parameters (the distance of the closest approach of ions a and the average ionic radius R±) were calculated by the formulas:
where R+ and R– are the radii of cation and anion. Cation size was assessed according to Pauling [14], while the radius of nitrate ion was assessed according to Marcus [15] (Table 2). Solution densities were taken from the handbook [16].
Thus, the calculation of activity coefficients was conducted without optimization of model parameters. The results of calculation of ion-average activity coefficient γ± at 298 K are shown in Fig. 2 in comparison with experimental data [17, 18]. The quantitative agreement is observed only for diluted solutions. For example, the deviation exceeds 1% for LiNO3 solutions at m > 0.4 mol/kg. In the remaining concentration range, the calculated ion-average activity coefficient is markedly larger than experimental value, the difference increasing on passing from LiNO3 to CsNO3. In accordance with the conclusions of the work [2], this fact indicates the contribution of ion association, whose role rises with cation radius.
The amplification of ion association in the series from NaNO3 to CsNO3 is confirmed by the analysis of electrical conductivity of solutions [19]. The quantitative description of activity coefficients in NaNO3 solutions until saturation solution concentration of 10.8 mol/kg was demonstrated by us in the work [4] using generalization for the considered model with inclusion of explicit allowance made for the formation of ion pairs. This description, however, had empirical and poorly predictive character because the obtained value of equilibrium constant only by order of magnitude corresponded to literature data, which, however, also show large scattering. Therefore, we are confined in this work only by the description results derived from the basic GDHT model [1].
At qualitative level, the calculation reproduces the correct order of curves for activity coefficients for salts with different cations: ion-average activity coefficient decreases on passing from LiNO3 to CsNO3. This is explained by both decrease of hydration contribution on the growth of cation radius (which is taken into account by the model) and by the amplification of ion association in the same series. Thus, the change in these two factors in this series of systems affects activity coefficients in the same direction. In this context, the solutions of alkali metal nitrates are similar to solutions of chlorides and iodides.
The feature of nitrate solutions that differs them from solutions of alkali metal chlorides and iodides consists in the leveling effect of nitrate ion on activity coefficients in solutions of K+, Rb+, and Cs+ ions. This effect is reproduced by the model and leads mainly to the leveling effect of nitrate ion on DC of the corresponding solutions (Fig. 1) and close radii for these ions. The second difference of nitrate series (beginning from NaNO3) consists in the trend of ion-average activity coefficient to monotonic decrease with concentration instead of formation of minimum. This feature in general case can be reproduced probably by the explicit allowance for ion association.
CONCLUSIONS
The results of calculations of activity coefficients for water and ions in solutions of alkali metal nitrates in the context of generalized Debye–Hückel theory explain the character of change in activity coefficients depending on cation radius and reflect the leveling effect of potassium, rubidium, and cesium cations on activity coefficients in the presence of nitrate ions. The revealed features may be used for the development of methods for the prediction of thermodynamic properties of electrolyte solutions, including nitrate ion containing systems [20] of technological value.
REFERENCES
I. Yu. Shilov and A. K. Lyashchenko, J. Phys. Chem. B 119, 10087 (2015). https://doi.org/10.1021/acs.jpcb.5b04555
I. Yu. Shilov and A. K. Lyashchenko, J. Mol. Liq. 240, 172 (2017). https://doi.org/10.1016/j.molliq.2017.05.010
I. Yu. Shilov and A. K. Lyashchenko, Russ. J. Inorg. Chem. 65, 1240 (2020). https://doi.org/10.1134/S003602362008015X
I. Yu. Shilov and A. K. Lyashchenko, J. Solution Chem. 48, 234 (2019). https://doi.org/10.1007/s10953-019-00860-8
I. Yu. Shilov and A. K. Lyashchenko, Russ. J. Inorg. Chem. 64, 1186 (2019). https://doi.org/10.1134/S0036023619090213
R. Buchner and G. Hefter, Phys. Chem. Chem. Phys. 11, 8984 (2009). https://doi.org/10.1039/B906555P
A. Lyashchenko and A. Lileev, J. Chem. Eng. Data 55, 2008 (2010). https://doi.org/10.1021/je900961m
J. Barthel, F. Schmithals, and H. Behret, Z. Phys. Chem. 71, 115 (1970). https://doi.org/10.1524/zpch.1970.71.1_3.115
J. Barthel, R. Buchner, and M. Münsterer, Electrolyte Data Collection, Pt. 2 (Dechema, Chemistry Data Series, Franfurt am Main, 1995).
Z. A. Filimonova, A. S. Lileev, and A. K. Lyashchenko, Russ. J. Inorg. Chem. 47, 1890 (2002).
A. S. Lileev, Z. A. Filimonova, and A. K. Lyashchenko, J. Mol. Liq. 103–104, 299 (2003). https://doi.org/10.1016/S0167-7322(02)00148-4
W. Wachter, W. Kunz, R. Buchner, and G. Hefter, J. Phys. Chem. A 109, 8675 (2005). https://doi.org/10.1021/jp053299m
U. Kaatze, J. Chem. Eng. Data 34, 371 (1989). https://doi.org/10.1021/je00058a001
L. Pauling, J. Am. Chem. Soc. 49, 765 (1927). https://doi.org/10.1021/ja01402a019
Y. Marcus, Ions in Solution and Their Solvation (Wiley, Hoboken, New Jersey, 2015).
I. D. Zaitsev, G. G. Aseev, Physicochemical Properties of Binary and Multicomponent Solutions of Inorganic Substances (Khimiya, Moscow, 1988) [in Russian].
W. J. Hamer and Y.-C. Wu, J. Phys. Chem. Ref. Data 1, 1047 (1972). https://doi.org/10.1063/1.3253108
Y. C. Wu and W. J. Hamer, J. Phys. Chem. Ref. Data 9, 513 (1980). https://doi.org/10.1063/1.555621
M.-C. Justice, R. Bury, and J.-C. Justice, Electrochim. Acta 16, 687 (1971). https://doi.org/10.1016/0013-4686(71)85037-5
A. S. Malyutin, N. A. Kovalenko, and I. A. Uspenskaya, Russ. J. Inorg. Chem. 65, 781 (2020). https://doi.org/10.1134/S0036023620050149
Funding
This study was financially supported by the Russian Foundation for Basic Research (project no. 19-03-00033) and by the State assignment for basic research for the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Shilov, I.Y., Lyashchenko, A.K. Analysis of Activity Coefficients in Aqueous Solutions of Alkali Metal Nitrates on the Basis of Dielectric Properties. Russ. J. Inorg. Chem. 66, 1036–1039 (2021). https://doi.org/10.1134/S0036023621070123
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621070123