Abstract
Phase equilibria in the copper–palladium system are extrapolated to absolute zero of temperature subject to the third law of thermodynamics. The continuous solid solution with a FCC structure that crystallizes from melt experiences ordering to yield two intermetallic phases (Kurnakov phases), whose compositions shrink to CuPd and Cu3Pd stoichiometries as temperature lowers. As temperature raises, palladium partially occupies copper sites in the intermetallic phases. Ordering obeying a second-order phase transition to yield a phase whose idealized composition may be formulated as Cu21Pd7. When in a solid state, the system forms an eutectoid at 390°C and 30 at % Pd and two dystectoids with coordinates of 510°C, 15 at % Pd and 600°C, 40 at % Pd.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The copper–palladium system has been extensively researched for decades [1–8]. In addition to its fundamental importance, its alloys are attractive as efficient catalysts [9–14]. Intermetallic compound CuPd is one of the most promising materials for the manufacture of hydrogen-selective membranes [15, 16].
The components of the Cu–Pd system form continuous solid solutions when the melt solidifies. Both copper and palladium crystallize in face-centered cubic (FCC) structures with insignificantly differing unit cell parameters. As temperature lowers, alloys experience ordering to yield intermetallic phases. Such the phases, first discovered by Kurnakov with coworkers in the copper–gold system [17–19], are usually called Kurnakov phases. Experimental data obtained by different researchers for the Cu–Pd systems in the range 300–600°C are significantly divergent. An essential reason for this divergence is the difficulty of equilibration at such the low temperatures. The equilibration time, which is determined by diffusion, increases exponentially as temperature lowers [20–23]. From this, it flows that, for each system, there is a temperature below which phase equilibrium data cannot be gained experimentally.
Relevant information can be gained from thermodynamic simulations. A plain option is the direct use of the third law of thermodynamics (which reads that the entropy of a system in equilibrium tends to zero as the temperature tends to absolute zero [24]).
In accordance with the third law of thermodynamics, all phases of variable composition (solid solutions and nonstoichiometric phases) must either decompose into components or other phases of constant composition or pass into a strictly ordered state [23, 25, 26]. The use of these postulates makes it possible to confidently extrapolate phase diagrams to absolute zero of temperature. The results will be reliable only when reliable experimental data are used corresponding to the equilibrium state of the system at the temperatures studied. We demonstrated the fruitfulness of this approach for the copper–gold example system [18].
Here, our goal was to apply this approach to the copper–palladium system.
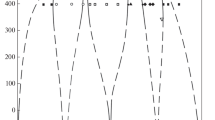
Our suggested phase diagram of the copper–palladium system is shown in Fig. 1. Liquidus and solidus curves are drawn in accordance with Subramanian and Laughlin’s data [7]. Phase equilibria in the solid solution ordering area at 300–450°C were thoroughly studied by a set of methods with controlled equilibration at the Nikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences [8]. We used these data in constructing the phase diagram. Popov et al.’s data [8] can be naturally extrapolated to zero temperature. All segments of curves drawn by continuous lines in [8] appear in Fig. 1.
In contrast, Subramanian and Laughlin’s data [7] does not allow for this type of extrapolation. This is especially true for the line of the limiting concentration of a disordered solid solution below 400°С. Thus, an indirect conclusion can be drawn about the reliability of Popov et al.’s data [8].
What is crystallized from melts in the Cu–Pd system is continuous solid solutions of the FCC structure (space group Fm\(\bar {3}\)m). The palladium distribution coefficient upon copper crystallization is close to unity, so with low palladium contents, the liquidus and solidus curves practically merge and run almost horizontally (Fig. 1).
The continuous solid solutions experience ordering to yield intermetallic phases. According to their X-ray diffraction patterns, these phases have CsCl and Cu3Au type structures of space group Pm\(\bar {3}\)m [3–8]. The ideal compositions corresponding to the complete differentiation of metal atoms over crystallographic positions correspond to the formulas CuPd and Cu3Pd. It is to these compositions that the homogeneity regions of two phases of variable composition shrink as the temperature tends to absolute zero. Rising temperature gives rise to the appearance of homogeneity regions of these phases; judging from their chemical compositions (enriched in copper), copper frequently occupies palladium sites in the intermetallic phases. It is noteworthy that the Cu–Pd systems, as opposed to the Cu–Au system, does not form a third (depleted in copper) intermetallic phase of ideal composition CuPd3.
Further, the phase diagram shows ordering by the second-order phase transition mechanism to yield a phase of ideal composition Cu21Pd7 (space group P4mm, a tetragonal lattice, one-dimensional ordering with a sevenfold increase in parameter с). One more ordered phase in the region of about 40 at % Pd at 400°C, observed by Subramanian and Laughlin [7], was not detected by Popov et al. [8], so is not shown in Fig. 1. This area of the phase diagram needs to be studied further.
An intriguing feature of our phase diagram version is the absence of intermetallics that are analogues of the ordered phase existing in the copper–gold system, namely, a CuPd tetragonal phase and a CuPd3 cubic phase. In the copper–palladium system these phases are dismissed by a CuPd phase, which has another structure–building principle.
The thermodynamic model of the Cu–Pd phase diagram proposed by Li et al. [1] is at variance with the third law of thermodynamics. The reason for this lies in that Li et al.’s calculations included low-temperature experimental phase equilibrium data. Geng et al.’s ab initio simulations [2] give a correct qualitative description to the thermal behavior of the CuPd phase, but no quantitative agreement with experimental data is observed.
Altogether, when in a solid state, the system forms an eutectoid at 390°C and 30 at % Pd and two dystectoids with the coordinates 510°C, 15 at % Pd and 600°C, 40 at % Pd [8].
REFERENCES
M. Li, Z. Du, C. Guo, and C. Li, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 32, 439 (2008). https://doi.org/10.1016/j.calphad.2008.04.004
F. Geng, J. R. Boes, and J. R. Kitchin, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 56, 224 (2017). https://doi.org/10.1016/j.calphad.2017.01.009
S. Bärthlein, G. L. W. Hart, A. Zunger, et al., J. Phys. Condens. Matter 19, 032201 (2007). https://doi.org/10.1088/0953-8984/19/3/032201
A. Y. Volkov, O. S. Novikova, and B. D. Antonov, Inorg. Mater. 49, 43 (2013). https://doi.org/10.1134/S0020168512110167
O. S. Novikova, A. Y. Volkov, and J. Matthey, Technol. Rev. 58, 195 (2014). https://doi.org/10.1595/147106714X684579
P. Huang, S. Menon, and D. de Fontaine, J. Phase Equil. 12, 3 (1991). https://doi.org/10.1007/BF02663663
P. R. Subramanian and D. E. Laughlin, J. Phase Equil. 12, 231 (1991). https://doi.org/10.1007/BF02645723
A. A. Popov, Yu. V. Shubin, P. E. Plyusnin, et al., J. Alloys Compd. 777, 204 (2019). https://doi.org/10.1016/j.jallcom.2018.10.332
S. Shan, V. Petkov, B. Prasai, et al., Nanoscale 7, 18936 (2015). https://doi.org/10.1039/C5NR04535E
J. Wu, S. Shan, J. Luo, et al., ACS Appl. Mater. Interfaces 7, 25906 (2015). https://doi.org/10.1021/acsami.5b08478
V. Sanchez-Escribano, L. Arrighi, P. Riani, et al., Langmuir 22, 9214 (2006). https://doi.org/10.1021/la0616101
K. A. Goulas, S. Sreekumar, Y. Song, et al., J. Am. Chem. Soc. 138, 6805 (2016). https://doi.org/10.1021/jacs.6b02247
B. Yan, C. Wang, H. Xu, et al., ChemPlusChem 82, 1121 (2017). https://doi.org/10.1002/cplu.201700245
F. Cai, L. Yang, S. Shan, et al., Catalysts 6, 96 (2016). https://doi.org/10.3390/catal6070096
V. V. Krisyuk, Y. V. Shubin, F. Senocq, et al., J. Cryst. Growth 414, 130 (2015). https://doi.org/10.1016/j.jcrysgro.2014.09.032
C. Zhao, A. Goldbach, and H. Xu, J. Membr. Sci. 542, 60 (2017). https://doi.org/10.1016/j.memsci.2017.07.049
N. S. Kurnakov, S. F. Zemczuzny, and M. M. Zasedatelev, J. Inst. Met. (London) 15, 305 (1916).
P. P. Fedorov and S. N. Volkov, Russ. J. Inorg. Chem. 61, 772 (2016). https://doi.org/10.1134/S0036023616060061
A. A. Smirnov, The Molecular Kinetic Theory of Metals (Nauka, Moscow, 1966) [in Russian].
P. P. Fedorov, Russ. J. Inorg. Chem. 37, 973 (1992).
M. Liu and R. A. Yund, Am. Mineral. 77, 275 (1992).
M. Yashima, M. Kakihana, and M. Yoshimura, Solid State Ionics 86–88, 1131 (1996).
P. P. Fedorov, Russ. J. Inorg. Chem. 55, 1722 (2010). https://doi.org/10.1134/S0036023610110100
W. Nernst, The New Heat Theorem (Methuen, London, 1917).
J. P. Abriata and D. E. Laughlin, Prog. Mat. Sci. 49, 367 (2004).
D. E. Laughlin and W. A. Soffa, Acta Mater. 145, 49 (2018). https://doi.org/10.1016/j.actamat.2017.11.037
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Fedorov, P.P., Shubin, Y.V. & Chernova, E.V. Copper–Palladium Phase Diagram. Russ. J. Inorg. Chem. 66, 891–893 (2021). https://doi.org/10.1134/S0036023621050053
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621050053