Abstract
The Knudsen effusion method with mass-spectral analysis of the gas phase has been used to study the vaporization processes in the PbO–ZnO system in the temperature range of 1010–1120 K. It has been found that the saturated vapor above the system consists of lead monooxide molecules, its associates, lead atoms, oxygen molecules, and mixed oxides PbZnO2, Pb2ZnO3, PbZn2O3, Pb2Zn2O4, and Pb3ZnO4. The molar composition of the saturated vapor has been determined and the partial pressures of all components of the gas phase have been obtained at 1110 K. The experimental data made it possible to calculate a number of standard enthalpies of heterophase reactions and standard enthalpies of formation of mixed oxides using the second law of thermodynamics: \({{\Delta }_{f}}H_{{{\text{298}}}}^{^\circ }\)(PbZnO2) = –290.4 ± 6.6 kJ/mol, \({{\Delta }_{f}}H_{{{\text{298}}}}^{^\circ }\)(Pb2ZnO3) = –488.2 ± 21.5 kJ/mol, \({{\Delta }_{f}}H_{{{\text{298}}}}^{^\circ }\)(PbZn2O3) = –628.4 ± 11.9 kJ/mol, \({{\Delta }_{f}}H_{{{\text{298}}}}^{^\circ }\)(Pb2Zn2O4) = –883.2 ± 15.1 kJ/mol, and \({{\Delta }_{f}}H_{{{\text{298}}}}^{^\circ }\)(Pb3ZnO4) = –697.1 ± 31.6 kJ/mol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Lead and zinc oxides are among the main components of many glasses with different properties and purposes, the optical, thermal, and mechanical characteristics of which significantly depend on their composition [1–3]. The composition of the charge can change uncontrollably during long-term high-temperature glass melting, not only due to the different volatility of the components but also due to the formation of complex oxides in the gas phase, which should inevitably lead to a change in the properties of the obtained glass. In this regard, high-temperature studies of the composition of saturated vapor over complex oxide systems and, in particular, over the PbO–ZnO system are highly relevant.
The purpose of this work is a mass-spectrometric study of the composition of the gas phase, the determination of the thermodynamic characteristics of the vaporization processes in the PbO–ZnO system, and the calculation of the standard enthalpies of formation of simple and complex oxides in saturated vapor.
EXPERIMENTAL
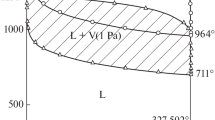
According to the data [4, 5], the phase diagram of the PbO–ZnO system has a simple eutectic with a composition of 95 mol % PbO and a temperature of 850–870°С. At the eutectic temperature, a slight dissolution of zinc oxide is observed (no more than 3 mol % in lead oxide). Thus, it can be assumed that below the eutectic temperature, the system is a mechanical mixture of zinc and lead oxides, the activity of which is practically equal to unity throughout the composition.
Solid-phase synthesis of samples with a composition of 10.0, 50.0, and 90.0 mol % PbO was carried out in platinum crucibles in air at a temperature of 1073 K for 35 h. The starting materials were PbO (yellow) and ZnO of the special quality grade (Russian State Standard) (Merck, Germany). The mixtures of oxides were ground with alcohol in a jasper mortar before starting annealing and several times, interrupting the annealing. The purity and phase composition of the synthesized samples were monitored by X-ray powder diffraction and X-ray fluorescence.
Vaporization in the system was investigated by the Knudsen effusion method with mass spectral analysis of the gas phase on an MS-1301 instrument. Evaporation was carried out in the temperature range 1010–1120 K. In this work, we used chambers made of zirconium oxide with alundum covers with a ratio of the evaporation area to the effective effusion area of ~200. The temperature was measured with a Pt–Pt/Rh thermocouple and kept constant with an accuracy of ±1°C. The mass spectrum of the gas phase was recorded at an ionizing voltage of 50–60 V.
RESULTS AND DISCUSSION
Composition of Gas Phase over the PbO–ZnO System
Table 1 shows the mass spectrum of the gas phase upon sublimation of the PbO–ZnO system with an initial composition of 50 mol % PbO.
Analysis of the mass spectrum and the study of ionization of saturated vapor molecules showed that in the gas phase over the PbO–ZnO system, in addition to lead oxide molecules and its associates (PbO)n, where n = 2–4, there are molecules of mixed oxides PbZnO2, PbZn2O3, Pb2ZnO3, Pb2Zn2O4, and Pb3ZnO4 as well as lead atoms. Considering that the process of vaporization of lead oxide proceeds congruently [6], there are also oxygen molecules in the gas phase. Moreover, under the conditions of an effusion experiment, the ratio of partial pressures should be \({{{{p}_{{{\text{Pb}}}}}} \mathord{\left/ {\vphantom {{{{p}_{{{\text{Pb}}}}}} {{{p}_{{{{{\text{O}}}_{{\text{2}}}}}}}}}} \right. \kern-0em} {{{p}_{{{{{\text{O}}}_{{\text{2}}}}}}}}}\) = 5.08. As shown by the study of ionization processes, the mass spectrum of saturated vapor is formed almost without deep fragmentation of molecules; for example, ions PbO+ are formed only from PbO molecules, ions \({\text{PbZnO}}_{{\text{2}}}^{ + }\)―from PbZnO2 molecules. Some exceptions are ions Pb2O+ and \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{2}}}^{ + },\) which are formed upon the dissociative ionization of Pb2O2 and Pb3O3 molecules, respectively. This is evidenced by the equality of the slopes obtained from the temperature dependences of the ion currents of Pb2О+, \({\text{P}}{{{\text{b}}}_{{\text{2}}}}{\text{O}}_{{\text{2}}}^{ + }\), and \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{2}}}^{ + },\) \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{3}}}^{ + }.\)
To determine the nature of vaporization, an experiment was carried out on isothermal sublimation of a sample in the PbO–ZnO system with an initial composition of 50 mol % PbO. As shown by measurements of ion current intensities (Table 1), all of them, with the exception of Pb+ and PbO+, remained constant during the entire period of vaporization until they completely disappeared. At the end of the experiment, according to X-ray powder diffraction data, zinc oxide remained in the effusion chamber as a non-volatile product. The results obtained are in agreement with the phase diagram [4, 5] and indicate that the activities of lead and zinc oxides are constant and close to unity in the process of sublimation of the PbO–ZnO system. Thus, the vaporization process in this system can be expressed by the following heterophase reactions:
The absolute values of partial pressures were calculated by preliminary calibration of the mass spectrometer according to the known value of the partial pressure of PbO molecules at Т = 1110 K, рPbO = 6.6 × 10–6 atm given in [6], atomic ionization cross sections [7], corrected additivity rule, and values of the intensity of ion currents of the mass spectrum obtained in the present work. The values of the partial pressures calculated in this way are given in Table 2.
Since the activity of lead oxide in the PbO–ZnO system is practically equal to unity, the composition of the gas phase and the values of the partial pressures of (PbO)n molecules should be the same as above pure lead oxide, which is consistent with most of the known literature data (Table 2), except for the recently published results [10]. According to that study [10], saturated vapor consists of lead atoms, oxygen molecules, and lead oxide PbO.
The found values of the partial pressures over the PbO–ZnO system were used to calculate the molar percentages of metals in the gas phase, which were found to be: n(Pb) = 94.0 mol %, n(Zn) = 6.0 mol %. It should be noted that this composition of the gas phase must be taken into account in high-temperature glass melting and slag processing in non-ferrous metallurgy.
Thermodynamic Characteristics of Simple and Complex Lead Oxides
In the course of studying the temperature dependences of the intensities of the main ion currents (partial pressures) of the mass spectrum of saturated vapor over the PbO–ZnO system (Table 1) in the temperature range 1010–1120 K using the Clausius–Clapeyron and van’t Hoff equations, the standard enthalpies of reactions (2), (3) were calculated by least-squares method. The enthalpy values obtained in this way and the values recalculated to T = 298.15 K are presented in Tables 3, 4.
The enthalpies of reactions (2) were referred to T = 298.15 K using the known heat capacities of solid lead oxide [13] and (PbO)n(g) molecules as estimated and reported in [8].
When referring the enthalpies of heterogeneous reactions (3) to T = 298.15 K, we used the known heat capacities of solid oxides of lead and zinc [14] and our estimates for complex oxide molecules. It was assumed that the heat capacity of the mixed oxide is equal to the heat capacity of a lead oxide molecule containing the same number of metal atoms; for example, ср(Pb2Zn2O4) = ср(Pb4O4).
The enthalpy of reaction (1) was found by studying the temperature dependence of its equilibrium constant in the range 1010–1120 K, which, taking into account the congruent vaporization of lead oxide, can be written as
or through the ion current \(I_{{{\text{Pb}}}}^{ + },\) corresponding to the ionization of only lead atoms:
where \({{p}_{{{\text{Pb}}}}}({{p}_{{{{{\text{O}}}_{{\text{2}}}}}}})\) is partial pressure of lead atoms (oxygen molecules) over lead oxide at temperature T and \(I_{{{\text{Pb}}}}^{ + }\) is the ionic current generated by ionization of lead atoms.
The calculation by the least squares method according to the van’t Hoff equation led to the value of the standard enthalpy of reaction (1) equal to \({{\Delta }_{r}}H_{T}^{^\circ }\)(1) = 409.6 ± 7.0 kJ/mol, while its value referred to T = 298.15 K according to [13] is 417.8 ± 7.0 kJ/mol.
From the found values of the standard enthalpies of reactions (1)–(3) and the known enthalpy of formation of zinc oxide [14], the standard enthalpies of formation of lead oxides PbO(c), (PbO)n(g), and complex compounds based on lead and zinc PbnZnmO(n + m)(g) oxides were calculated using the Hess law (Tables 4, 5).
As it is clear from Tables 3 and 5, the thermodynamic characteristics of lead oxides obtained by us are in good agreement with most of the literature data, with the exception of the results presented [10], according to which the gas phase above yellow lead oxide consists of lead atoms, oxygen molecules, and lead oxide PbO. The data obtained can only be associated with the experimental research methodology [10]. In their work carried out by the Knudsen effusion method with mass spectral analysis of the gas phase, the authors used the Knudsen iridium cell. Undoubtedly, iridium is the best material for Knudsen cells in the study of chemically active compounds, in particular metal oxides, but only if the saturated vapor above the compound under study contains no metal atoms or molecules. Otherwise, the latter will dissolve at high temperatures in the material (iridium, platinum) of the Knudsen chambers. This process, as noted many times, leads to noticeable distortions in the composition of saturated vapor [16–18] and in the thermodynamic characteristics of the compounds to be studied. In addition, this situation leads to the fact that the cell material can no longer be considered as an individual inert metal, since its properties can be determined by the chemical activity of the dissolved metal. According to [10], the iridium chamber was used to study various oxides, including oxides of alkali metals, namely, sodium oxide in the temperature range 1000–1100 K [19]. Based on the publication date of work [19], it can be assumed that the study of Na2O took place in the same period as the study of PbO, and could even have preceded it. The vaporization of Na2O proceeds with the transition to the gas phase of sodium atoms and oxygen molecules [20, 21]:
It is highly probable that under these conditions the iridium effusion chamber will contain dissolved sodium, the activity of which, taking into account the mass of the chamber and the investigated Na2O sample, should not exceed 0.001–0.0005. This assumption is consistent with the results of [22, 23], where the interaction of zinc vapor with metallic platinum and gold was studied using the isopiestic method. For example, upon interaction for 4–6 days in areas enriched in platinum and gold, the activity of dissolved zinc at a temperature of 1173 K was аZn(Pt) = 0.016 and аZn(Au) = 0.04. According to the Ellingham diagram [24], in the temperature range 273–1800 K sodium is a strong reducing agent in reactions with lead oxide:
Calculation of the Gibbs energy of this reaction at Т = 1110 K and аPbO = 1, аNa = 0.001, \({{a}_{{{\text{N}}{{{\text{a}}}_{{\text{2}}}}{\text{O}}}}}\) = 1, and РPb = 3.2 × 10–5 atm leads to a negative value \(\Delta G_{{{\text{111}}0}}^{^\circ }\) = –32.0 kJ/mol. The result obtained allows us to give a completely reasonable explanation of Kobertz’s results [10], which consists in a high content of lead atoms and the absence of polymer molecules in saturated vapor. The initially high intensities of the ionic current of Pb+ in our studies (Table 1) are most likely associated with similar reasons, namely, an insignificant impurity of sodium oxide in the ceramic effusion chamber, the rapid burnout of which is accompanied by the establishment of an equilibrium vapor over the lead oxide.
It is necessary to note some difference between the works of Popovic [8] and Knacke [9] on the one hand and Semenikhin [15], Drowart [6], Kazenas [11] and this study, on the other, associated with the content of trimeric lead oxide (PbO)3 molecules. The first group of authors [8, 9] believe that these molecules are absent in measurable amounts in the gas phase of lead oxide, and ions \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{2}}}^{ + }\) and \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{3}}}^{ + }\) in the mass spectrum are formed due to the dissociative ionization of Pb4O4 molecules. This conclusion was made when analyzing the energies of ion appearance and ionization efficiency curves. Using a similar approach and studying the temperature dependences of the intensities of the main ions in the mass spectrum, the authors of the second group [6, 11, 15] and this work came to the conclusion that the saturated vapor of lead oxide contains trimeric molecules. This assumption is supported by the results of a study of the processes of vaporization of lead oxide. As you can see from Table 3, the values of the enthalpies of sublimation of trimeric molecules calculated from ion currents of \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{2}}}^{ + }\) and \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{3}}}^{ + }\) are noticeably higher than the values of the enthalpies of sublimation of tetrameric molecules. This result unambiguously testifies to the presence of trimeric molecules in the saturated vapor, which mainly correspond to ions \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{2}}}^{ + }\) and \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{3}}}^{ + }\) in the mass spectrum. The authors [8, 9] made their conclusion, most likely, based on the relatively high value of the appearance energy (9.1 eV) of ion \({\text{P}}{{{\text{b}}}_{{\text{3}}}}{\text{O}}_{{\text{3}}}^{ + }\), considering it a fragmentation ion formed by dissociative ionization of tetrameric molecules.
CONCLUSIONS
As a rule, the reliability of experimental data is determined by the coincidence of the thermodynamic characteristics calculated according to the second and third laws of thermodynamics. Nevertheless, in this study, only the second law of thermodynamics was used when we calculated the standard enthalpies of heterophase reactions and the formation of lead oxides and mixed oxides according to experimental data,. This is because of the lack of reliable data on the molecular constants of oxide molecules in the gas phase. However, the correctness of this study can be judged by the good coincidence of the value of the standard enthalpy of formation of crystalline (yellow) lead oxide found in this work by the Knudsen method with those found by the EMF method [9, 25] and given in handbooks [26, 27].
The process of vaporization of the PbO–ZnO two-component system was studied using the Knudsen effusion method with mass-spectral analysis of the composition of the gas phase.
It was observed for the first time that in a saturated vapor, along with molecules of lead oxide and its associates, there are molecules of mixed oxides with the general formula PbnZnmО(n+)(g) (n = 1, 2, 3; m = 1, 2).
According to the second law of thermodynamics, the standard enthalpies of formation were calculated for the first time for five mixed oxides: PbZnO2(g), PbZn2O3(g), Pb2ZnO3(g), Pb2Zn2O4(g), Pb3ZnO4(g), lead oxides of the general formula (PbO)n(g), and PbO(c); the calculated values agree with most of the literature data.
REFERENCES
M. I. Sayyeda, Y. S. Rammahb, A. S. Abouhaswab, et al., Physica B: Condens. Mater. 548, 20 (2018). https://doi.org/10.1016/j.physb.2018.08.024
H. A. A. Sideka, S. H. Elazoumia, R. El-Mallawanyb, et al., J. Non-Cryst. Solids 523 (2019). https://doi.org/10.1016/j.jnoncrysol.2019.119640
H. Ticha, J. Schwarz, and L. Tichy, Mater. Chem. Phys. 237, 121834 (2019). https://doi.org/10.1016/j.matchemphys.2019.121834
E. Jak, S. A. Decterov, P. Wu, et al., Metall. Mater. Trans. B 28, 1011 (1997). https://doi.org/10.1007/s11663-997-0055-x
M. E. Shevchenko and E. Jak, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 64, 318 (2019). https://doi.org/10.1016/j.calphad.2019.01.011
J. Drowart, R. Colin, and G. Exsteen, Trans. Faraday Soc. 61, 1376 (1965). https://doi.org/10.1039/tf9656101376
J. B. Mann, J. Chem. Phys. 46, 1646 (1967). https://doi.org/10.1063/1.1840917
A. Popovic, A. Lesar, M. Gucek, and L. Bencze, Rapid Commun. Mass Spectrom. 11, 459 (1997). https://doi.org/10.1002/(sici)1097-0231(199703)11:5<459::aid-rcm889>3.0.co;2-g
O. Knacke and A. Richthoven, Z. Phys. Chem., No. 187, 257 (1994). https://doi.org/10.1524/zpch.1994.187.part_2.257
D. Kobertz, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 65, 155 (2019). https://doi.org/10.1016/j.calphad.2019.02.012
E. K. Kazenas and A. A. Petrov, Metally, No. 4, 22 (1996).
S. I. Lopatin and I. Ya. Mittova, et al., Russ. J. Inorg. Chem. 51, 1646 (2006). https://doi.org/10.1134/S0036023606100214
Thermal Constants of Materials. Handbook, Ed. by V. P. Glushko (VINITI, 1965), Vol. IV, Book 2 [in Russian].
Thermal Constants of Materials. Handbook, Ed. by V. P. Glushko (VINITI, 1965), Vol. VI, Book 2 [in Russian].
A. N. Semenikhin, A. N. Rykov, and L. N. Sidorov, Zh. Fiz. Khim. 150, 1663 (1983).
N. A. Gribchenkova, A. B. Steblevsky, and A. S. Alikhanyan, J. Chem. Thermodyn. 1151, 6 (2017). https://doi.org/10.1016/j.jct.2017.07.009
N. A. Gribchenkova and A. S. Alikhanyan, J. Alloys Compd. 778, 77 (2019). https://doi.org/10.1016/j.jallcom.2018.11.136
K. G. Smorchkov, N. A. Gribchenkova, and A. S. Alikhanyan, Zh. Neorg. Khim. 65, 2020. https://doi.org/10.31857/S0044457X20110185
D. Kobertz, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 64, 327 (2019). https://doi.org/10.1016/j.calphad.2019.01.006
D. L. Hildenbrand and E. Murad, J. Chem. Phys. 53, 3403 (1970). https://doi.org/10.1063/1.1674508
M. Steinberg and K. A. Schofield, J. Chem. Phys. 94, 3901 (1991). https://doi.org/10.1063/1.460666
H. Sasaki, T. Nagai, and M. Maeda, J. Alloys Compd. 504, 475 (2010). https://doi.org/10.1016/j.jallcom.2010.05.146
H. Sasaki, M. Miyake, and M. Maeda, J. Electrochem. Soc. 157, E82 (2010). https://doi.org/10.1149/1.3332468
H. J. T. Ellingham, J. Soc. Chem. Industry. Trans. Commun., 125 (1944). https://doi.org/10.1002/jctb.5000630501
M. J. Bannister, J. Chem. Thermodyn. 16, 787 (1984). https://doi.org/10.1016/0021-9614(84)90063-6
D. D. Wagman, Nati. Bureau Standards Rep. 89, 8919 (1965).
JANAF Thermochemical Tables. Part II, J. Phys. Chem. Ref., Suppl. 1, 1643 (1985).
Funding
The work was carried out within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences in the field of fundamental research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Gribchenkova, N.A., Smorchkov, K.G., Smirnov, A.S. et al. Thermodynamics of Compounds Based on Lead(II) and Zinc(II) Oxides in Gas Phase. Russ. J. Inorg. Chem. 66, 385–390 (2021). https://doi.org/10.1134/S0036023621030098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023621030098