Abstract
The phase formation in the sections Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3 of the quasi-ternary system Ag2Se–SnSe2–Cu2Sе was studied for the first time by differential thermal analysis (with thermodynamic calculations), X-ray powder diffraction analysis, and microstructural analysis and also by microhardness and density measurements. No new quaternary compounds were detected. It was determined that both sections are quasi-binary and are of simple eutectic type with limited solubility based on the initial selenides. The coordinates of the eutectic points are (40 mol % Ag2Se, 910 K) and (50 mol % Ag8SnSe6, 780 K). The solubility based on Cu2SnSe3 was 10 mol % Ag2Se in the system Ag2Se–Cu2SnSe3 and 15 mol % Ag8SnSe6 in the system Ag8SnSe6–Cu2SnSe3. Single crystals of the compound Cu2SnSe3 and the solid solutions (Cu2SnSe3)1 – х(Ag8SnSe6)х (х = 0.02–0.15) were grown by the Bridgman–Stockbarger directional crystallization method. It was found that these solutions crystallize in the monoclinic system, and the unit cell parameters increase with increasing Ag8SnSe6 content: а = 6.968–6.985 Å, b = 12.051–12.078 Å, с = 6.945–6.968 Å, β = 109.20°–109.30°, space group Сс, Z = 4, and ρ = 5.75–5.86 g/cm3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Investigation of the interaction between semiconductor compounds, in particular, between silver and copper chalcogenides, which have superionic conduction, is of great scientific and practical interest. According to Ioffe’s concepts [1], the complication of the composition of solid solutions improves a number of physical properties, e.g., the thermoelectric figure of merit. In this context, it was of interest to study the phase equilibria in the systems Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3.
Selenides Ag2Se, Cu2SnSe3, and Ag8SnSe6 were investigated previously [2–21]. These compounds melt congruently at 1170, 973, and 1017 K, respectively. According to the published data [5–7, 12, 16, 17], Ag2Sе and Ag8SnSe6 undergoes a single polymorphic transition each at 410 and 356 K, respectively. The published data on the crystal structure of the low-temperature polymorph α-Ag2Se are contradictory [22, 23]. It was reported [24] that the low-temperature polymorph Ag2Se has a pseudocubic structure with the lattice parameter a = 4.978 Å, and the high-temperature polymorph β-Ag2Se crystallizes in the orthorhombic system with the unit cell parameters a = 7.067 Å, b = 7.80 Å, and c = 4.34 Å [25, 26], or a = 4.333 Å, b = 7.162 Å, c = 7.764 Å [5, 12] with the space group Р212121.
The low-temperature polymorph α-Ag8SnSe6 crystallizes in the cubic system (a = 11.12 Å), the high-temperature polymorph β-Ag8SnSe6 crystallizes in the orthorhombic system (a = 7.9168 Å, b = 7.8219 Å, c = 11.045 Å, space group Рmn21, and ρcalc = 7.072 g/cm3 [15–19]. This compound is isostructural to Сu8GeS6 and β-Ag8GeSе6 [17]. The average interatomic distance Ag–Se in the structure of Ag8SnSe6 is 2.59–2.82 Å.
The compound Cu2SnSe3 has a cubic lattice, the period of which varies (a = 5.688–5.696 Å) within the homogeneity region [15, 16, 27]. This compound has a monoclinic structure with the unit cell parameters a = 6.5936 Å, b = 12.1593 Å, c = 6.6084 Å, β = 108.56°, space group Сс [28].
The initial compounds Ag2Se, Ag8SnSe6, and Cu2SnSe3 attract researchers’ attention as promising materials for using in nonlinear optical devices and photoelements [29–34], in view of which the investigation of the interaction between them are of both theoretical and practical importance.
EXPERIMENTAL
The initial compounds and a set of alloys were prepared for the experiments. Ag2Se, Ag8SnSe6, and Cu2SnSe3 were synthesized from high-purity selenium, high-purity electrical copper, and silver (>99.999%). The compounds were obtained by alloying elemental components weighed with an accuracy of 0.0001 g. The alloying was carried out in sealed evacuated (to 10–3 mm Hg) quartz ampules. The temperature was increased slowly with 1-h-long holdings at the melting points of Ag2Se, Ag8SnSe6, and Cu2SnSe3 (1170, 1017, and 973 K, respectively). After the completion of the synthesis, a 2–3-h-long holding was performed at 1060 K for Ag2Se, 920 K for Ag8SnSe6, and 900 K for Cu2SnSe3. The cooling was carried out in a turned -off furnace. To study the phase equilibria in each of the systems Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3, 11 alloys of various compositions at an interval of 5–10 mol % were synthesized from the obtained initial compounds by alloying in sealed evacuated ampules with 40–45-min-long holding at temperatures 50–60°C above the melting points. The alloys were quenched in water and then were subjected to homogenizing annealing for 250–340 h at 740 K for the system Ag8SnSe6–Cu2SnSe3 and 850 K for the system Ag2Se–Cu2SnSe3.
The alloys were studied by differential thermal analysis, X-ray powder diffraction analysis, and metallography. The differential thermal analysis was performed with a Kurnakov pyrometer using a chromel–alumel thermocouple. The heating and cooling curves were recorded for each sample. The heating rate was 8–10 deg/min, the cooling was carried out in a turned-off furnace. The solidus points were determined at the beginning of the deviation of the differential curve in the heating curve; and the solidus points, at the beginning of the deviation of the differential curve in the cooling curve. The liquidus temperature found from the cooling curves was controlled by the maximum deviation of the differential curve during heating, and the solidus temperature obtained from the heating curves was controlled by the maximum deviation during cooling. The sample weight was 2–3 g.
The X-ray powder diffraction analysis was made with a Bruker D2 РILSENER (CuKα radiation, Ni filter, scan angle range 10° ≤ 2θ ≤ 90°). The microhardness of the samples was measured with a PMT-3 microhardness meter (the optimal load was 0.02 kg), and the microstructure of samples was investigated with a MIM-7 microscope with diluted aqueous nitric acid solution as an etchant. The error of measuring the heats was ±2°, the error of calculating the unit cell parameters was ±0.001 Å, and the error of measuring the microhardness was ±0.005 MPa.
RESULTS AND DISCUSSION
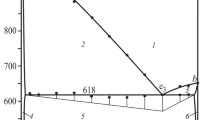
The T–x diagrams of the systems Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3 were constructed using the differential thermal analysis data (Figs. 1, 2). As Fig. 1 shows, the section Ag2Se–Cu2SnSe3 is of the eutectic type. The coordinates of the eutectic point are (40 mol % Ag2Se, 910 K). The solubility based on Cu2SnSe3 at the eutectic temperature is 15 mol % Ag2Se, and that at 300 K is 10 mol % Ag2Se. The solubility based on silver selenide was not virtually detected. The difference of the ionic and atomic radii of Cu+ and Ag+ is 8.87%, which is within 15% dictated by the Hume-Rothery rule [35]. Although the geometric parameters are close, the mutual solubility range is narrow, which is likely to be related to the difference between the crystal structures of Ag2Se (cubic) and Cu2SnSe3 (monoclinic). The liquidus of the section comprises the branches of primary crystallization of β-Ag2Se and γ-solid solutions based on Cu2SnSe3. The phase transition α-Ag2Se \( \rightleftarrows \) β-Ag2Se occurs at 410 K. The phase formation in the section Ag8SnSe6–Cu2SnSe3 is similar. As Fig. 2 shows, the section is a quasi-binary section of the quasi-ternary system Ag2Se–SnSe2–Cu2Sе and is of the eutectic type. The coordinates of the eutectic point is (50 mol % Cu2SnSe3, 780 K). The mutual solubility between the components is limited: 15 mol % Ag8SnSe6 based on Cu2SnSe3 and 5 mol % Cu2SnSe3 based on cubic Ag8SnSe6. The temperature of the phase transition α-Ag8SnSe6 \( \rightleftarrows \) β-Ag8SnSe6 decreases with increasing Cu2SnSe3 content; i.e., the transformation is eutectoid.
For the microscopic examination, flat samples were cut from the synthesized alloys. The surface of the samples was initially ground and then polished with a diamond paste. The polished layer was removed from the surface by etching with 50% HNO3 + 50% H2O to open the microstructure of the material. The micrographs of the surfaces of each sample were taken with a MIM-7 reflecting-type metallographic microscope. The micrographs of the surfaces of the (Cu2SnSe3)1 – х(Ag8SnSe6)х samples (Fig. 3) demonstrate that all the obtained alloys are single-phase and have large-block structures without any inclusions. The structure, sizes, and positions of grains differ in different samples. Single crystals of the solid solutions (Cu2SnSe3)1 – х(Ag8SnSe6)х (х = 0.02 and 0.15) were grown by the Bridgman–Stockbarger directional crystallization method. For this purpose, the synthesized polycrystalline samples with the composition Cu1.98Ag0.02SnSe3 and Cu1.85Ag0.15SnSe3 were placed in 12-cm-long ampules 0.5 cm i.d.. The evacuated (to 10–3 mm Hg) ampules with the samples were moved within a vertical furnace with two temperature zones. In the upper zone, the temperature (T1) was maintained 50–60 K above the melting point of the sample with a certain composition, and in the lower zone, the temperature (T2) was kept 50–60 K below this melting point. In the upper zone, the sample in the ampule are in the molten state. Once the tip of the ampule passes through the melting point, in the lower zone, Cu1.98Ag0.02SnSe3 Cu1.85Ag0.15SnSe3 crystal nuclei form. The motion of the ampule at a velocity of 0.2–0.3 cm/h was suitable for growing the formed crystal nuclei of the samples. Figure 4 shows the thus grown single crystals of Cu1.98Ag0.02SnSe3 and Cu1.85Ag0.15SnSe3, which are suitable for single-crystal X-ray diffraction and physical studies. The formation of a limited solubility region in the system Ag8SnSe6–Cu2SnSe3 was also confirmed by the X-ray powder diffraction data (Fig. 5). It was determined that the X-ray powder diffraction patterns of the alloys containing ≤15 mol % Ag8SnSe6 are qualitatively identical to that of Cu2SnSe3; i.e., these alloys are substitutional solid solutions based on this compound (α-phase), whereas the X-ray powder diffraction patterns of the alloys containing ≤5 mol % Cu2SnSe3 are similar to those of pure Ag8SnSe6 (β-phase). The X-ray powder diffraction patterns of the alloys containing 5–85 mol % Cu2SnSe3 comprise the lines of the α- and β-phases, which is consistent with the phase diagram (Fig. 2). The X-ray powder diffraction patterns of the α-phase were indexed in the monoclinic system (Table 1). Within the homogeneity region of the α-phases, the concentration dependences of their unit cell parameters are virtually linear.
THERMODYNAMIC ANALYSIS
The boundaries of the solid solutions formed in the quasi-binary sections Ag2Se–Cu2SnS3 and Ag8SnSe6–Cu2SnSe3 were refined using the dependence of the Gibbs free energy on temperature and concentration. An analysis of the published data showed that the compounds Ag2Se, Cu2SnS3, and Ag8SnSe6 differ significantly in composition, structure, and unit cell parameters. Therefore, in the Gibbs–Helmholtz equation, an asymmetric variant of the model of regular solid solutions of nonmolecular compounds was used [36–39]. In particular, the Gibbs–Helmholtz equations for the solid solutions (1 – x)Ag8SnSe6 – xCu2SnSe3 and (1 – x)Ag2Se–xCu2SnSe3 have the form
In Eqs. (1) and (2), x is the mole fraction of Cu2SnSe3 in the solid solutions (1 – x)Ag8SnSe6–xCu2SnSe3 and (1 – x)Ag2Se–xCu2SnSe3; R = 8.314 J mol–1 K–1; and \(\Delta H_{{298}}^{0}\) and \(\Delta S_{{298}}^{0}\) are the standard enthalpies and entropies of formation of compounds, respectively. Their values were taken from the literature [40, 41]: \(\Delta H_{{298}}^{0}\)(Ag8SnSe6) = –207.6 kJ mol–1, \(\Delta H_{{298}}^{0}\)(Cu2SnSe3) = –320.4 kJ mol–1, \(\Delta S_{{298}}^{0}\)(Ag8SnSe6) = –31.33 J mol–1 K–1, and \(\Delta S_{{298}}^{0}\)(Cu2SnSe3) = 100.35 J mol–1 K–1. axm(1 – x)n represents the enthalpies of mixing of solid solutions in the asymmetric variant of the model of regular solid solutions. The constants in this formula were found by a multipurpose genetic algorithm [42]. Iterations were made under the following conditions together with the experimental data of the differential thermal analysis:
The last terms in Eqs. (1) and (2) represent the configurational entropy of mixing (Т∆S) of the solid solutions with taking into account the nonmolecular nature of the compounds Ag8SnSe6, Cu2SnSe3, and Ag2Se. The stoichiometric coefficients of unlike atoms in the molecules of the compounds are included as constants in the functions f1(x) [43].
In particular, Eq. (1) after substituting the values of the thermodynamic quantities and the parameters of the model of regular solid solutions takes the form
Figure 6 illustrates the dependences of the Gibbs free energy of formation of the solid solutions (1 – x)Ag8SnSe6– xCu2SnSe3 on the composition at temperatures of 325 and 780 K. Figure 6 suggests that, at medium concentrations, the solid solutions are unstable and break up into two phases. The homogeneity regions were determined from the position of the common tangent in Fig. 6 based on the equality of the chemical potentials of a component in the heterogeneous mixture of the solid solutions of two different types (Figs. 1 and 2). The calculations were performed and visualized using the software OriginLab2019 and www.matematikam.ru.
Dependences of the Gibbs free energy of formation of solid solutions on the composition at various temperatures (Eq. (3)).
CONCLUSIONS
The T–x phase diagrams of the quasi-binary sections Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3 of the quasi-ternary system Ag2Se–SnSe2–Cu2Sе were constructed for the first time. It was determined that their state diagrams are of the eutectic type with limited solubility based on the components. The dependences of the Gibbs free energy of alloys on temperature and concentration were characterized, which enabled one to refine the boundaries of the solid solutions (1 – x)Ag8SnSe6–xCu2SnSe3: 15 mol % Ag8SnSe6 based on Cu2SnSe3 and 12 mol % Cu2SnSe3 based on orthorhombic Ag8SnSe6.
The constructed phase diagrams were used for choosing the composition of melt solutions growing single crystals of the α-phase (solid solution based on Cu2SnSe3) and the β-phase (solid solution based on Ag8SnSe6) of a given composition by directional crystallization.
REFERENCES
A. F. Ioffe, Physics of Semiconductors (Akad. Nauk SSSR, Moscow, 1957) [in Russian].
N. Kh. Abrikosov, V. F. Bankina, L. V. Poretskaya, et al., Semiconductor Chalcogenides and Alloys Based on Them (Nauka, Moscow, 1975) [in Russian].
Physicochemical Properties of Semiconductors, Ed. by L. V. Novoselova and V. B. Lazareva (Nauka, Moscow, 1979) [in Russian].
M. Kh. Balapakov, R. A. Yakshibaev, and U. Kh. Mukhammed’yanov, Fiz. Tverd. Tela 45, 604 (2003).
M. B. Babanly, Yu. A. Yusibov, and V. T. Abishev, Ternary Chalcogenides Based on Copper and Silver (BGU, Baku, 1993) [in Russian].
E. Shimato and N. Okasaki, J. Phys. Condens. Mater. 5, 3405 (1993).
N. N. Bakkulova, Doctoral Dissertation in Mathematics and Physics (Ufa, 2005).
Y. S. Tveryanovich, A. A. Razumtcev, T. R. Fazletohinov, et al., Thin Solid Films 666, 172 (2018). https://doi.org/10.1016/j.tsf./2018.09.036
Qi Cao, Yi-Feng Cheng, Han Bi, et al., J. Mater. Chem. A 3, 20091 (2015). https://doi.org/10.1039/C5TA049780
P. J. Prathiba, K. H. Mohanraj, and G. Siva Kumar, Trans. Nonferrous Met. Soc. 25, 2241 (2015). https://doi.org/10.1039/C5TA049780
N. A. Okereke and A. I. Ekounobi, J. Optoelectron. Biomed. Mater., No. 3, 51 (2011).
Yu. A. Yusibov, I. Dzh. Alverdiev, L. F. Mashadiyeva, et al., Russ. J. Inorg. Chem. 63, 162 (2018). https://doi.org/10.1134/S0036023618120227
M. Kreutzbruck, B. Mocwitz, and F. Gruhl, J. Appl. Phys. Lett. 86, 813 (2005).
U. M. Chougale, S. H. Han, M. C. Rath, et al., Mater. Phys. Mech. 17, 47 (2013).
I. D. Olekseyuk, T. A. Ostapyuk, and T. V. Yuhimuk, Nauk. Vislik Volinsk. Nats. Univ. Imeni Leci Ukrainki. Rozdil I. Neorg. Khim. 29, 35 (2009).
G. E. Delgado, A. J. Mora, G. Marcano, et al., Mater. Res. Bull. 38, 1949 (2003).https://doi.org/10.1016/j.materresbull.2003.09.017
L. D. Gulay, I. D. Olekseyuk, and O. V. Parasyuk, J. Alloys. Compd. 339, 113 (2002). https://doi.org/10.1016/S0925-8388(01)01970-3
V. A. Rzaguliev, O. Sh. Kerimli, and Sh. G. Mamedov, Proc. XXI Intern. Conf., St. Petersburg, 2019, p. 20.
V. A. Rzaguliev, O. Sh. Kerimli, D. S. Azhdarova, et al., Kondens. Sredy Mezhfaznye Granitsy 21, 544 (2019). https://doi.org/10.17308/komf.2019.21/2365
V. V. Rakitin, Candidate’s Dissertation in Chemistry (Moscow, 2016).
O. F. Zmiy, L. D. Gulay, and O. S. Klimovich, Chem. Met. Alloys, No. 1, 115 (2008). www.chemicaljournal.org.
O. S. Klymovch, Chem. Met. Alloys, No. 1, 288 (2008).
J. Yu and H. Yun, Acta Crystallogr. E 67, 145 (2011).
H. Liu, Nat. Mater. 11, 422 (2012).
L. W. Constantinesch, Thin Solid Films 28, 73 (1983).
Yu. G. Asadov, Yu. I. Aliev, and A. G. Babaev, Fiz. Elem. Chastits At. Yadra 46, 812 (2015).
B. B. Sharma, R. Ayyar, and H. Shing, Phys. Status Solidi A 40, 691 (1977).
G. Marcano, L. M. Chalbaud, C. Rincon, et al., Mater. Lett. 53, 151 (2002).
D. Avellaneda, M. T. Nair, and P. K. Nair, J. Thermochem. Soc. 158, 346 (2010).
G. Gurieva, S. Levchenko, S. Schorr, et al., Thin Solid Films 535, 384 (2013).
K. M. Kim, H. Tampo, and H. Shibata, Thin Solid Films 536, 11 (2013).
Zh. I. Alferov, Usp. Khim. 82, 587 (2013).
M. Meitin, Elektron.: Nauka, Tekhnol. Biznes, No. 6, 40 (2000).
Zh. I. Alferov, M. Andreev, and V.D. Rumyantsev, Fiz. Tekh. Poluprovodn. 38, 937 (2004).
W. Hume-Rothery, Atomic Theory for Students of Metallurgy (Inst. of Metals, London, 1955).
G. R. Gurbanov, Sh. G. Mamedov, M. B. Adygezalova, et al., Russ. J. Inorg. Chem. 62, 1638 (2017). https://doi.org/10.1134/S0036023617120099
Sh. H. Mammadov, A. N. Mammadov, and R. C. Kurbanova, Russ. J. Inorg. Chem. 65, 217 (2020). https://doi.org/10.1134/S003602362001012X
A. N. Mamedov, E. R. Tagiev, and M. B. Babanly, Inorg. Mater. 52, 543 (2016). https://doi.org/10.1134/S002016851606008X
S. M. Asadov, S. N. Mustafaeva, and A. N. Mamedov, J. Therm. Anal. Calorim. 133, 1135 (2018). https://doi.org/10.1007/s10973-018-6967-7
M. B. Babanly and Yu. A. Yusibov, Electrochemical Methods in Thermodynamics of Inorganic Systems (ELM, Baku, 2011) [in Russian].
M. V. Moroz, M. V. Prokhorenko, P. Yu. Demchenko, et al., J. Chem. Thermodyn. 106, 228 (2017).
A. N. Mammadov, I. D. Alverdiev, Z. S. Aliev, et al., Adv. Intell. Syst. Comput. 1095, 8 (2020). https://doi.org/10.1007/978-3-030-35249-3_118
A. N. Mamedov, Thermodynamics of Systems with Nonmolecular Compounds: Calculation and Approximation of Thermodynamic Functions and Phase Diagrams (Lambert Academic Publishing, Saarbrücken, Germany, 2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Rzaguliev, V.A., Mamedov, A.N., Kerimli, O.S. et al. Phase Equilibria in the Ag2Se–Cu2SnSe3 and Ag8SnSe6–Cu2SnSe3 Systems. Russ. J. Inorg. Chem. 65, 1899–1904 (2020). https://doi.org/10.1134/S003602362012013X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362012013X