Abstract
New iron(II) complexes with 2,6-bis(benzimidazol-2-yl)pyridine (L) and closo-borate(2–) anions—[FeL2][B10H10] ⋅ 2H2O and [FeL2][B12H12] ⋅ H2O—have been synthesized. The complexes have been characterized by static magnetic susceptibility measurements, electronic (diffuse reflectance spectra), IR, and EXAFS spectroscopy, and X-ray diffraction analysis. A study of the μeff(T) dependence in the temperature range 80–500 K has shown that the complexes exhibit a high-temperature spin crossover 1А1 ⇔ 5Т2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Spin crossover (SCO, spin transition) can manifest itself in 3d4–3d7 metal complexes with an octahedral or pseudo-octahedral environment of ligands of a certain field strength. The change in the spin multiplicity occurs in response to external stimuli: change in temperature and pressure, irradiation with light of a certain wavelength, and other factors. Monographs and numerous reviews and articles [1–9] have been devoted to the study of this phenomenon. Compounds capable of existing in two states with a sufficiently long lifetime are promising for use as active elements in electronic devices [3, 10–15]. One of the classes of compounds in which a spin crossover is observed includes octahedral iron(II) complexes with polynitrogen-containing heterocyclic ligands forming the FeN6 coordination core. In a number of such compounds, thermally induced SCO is observed. The temperature and nature of the spin transition in these complexes depend on a number of factors, in particular, on their structure, the nature of the ligand and anion, the presence and number of crystallization solvent molecules, e.g., water, in the structure. It should be especially noted that the SCO temperature significantly depends on the anions occupying the outer-sphere position in these complexes.
In the present work, 2,6-bis(benzimidazol-2-yl)pyridine (L) (Scheme 1) has been used as a ligand for the synthesis of complexes. Before the beginning of our work, only a few iron(II) compounds with L were known to possess SCO, in particular, Fe(II) perchlorate and tetraphenylborate complexes of [16, 17]. We have synthesized a number of Fe(II) complexes with L of the composition [FeL2]Ai · nH2O (A = \({\text{SO}}_{{\text{4}}}^{{{\text{2}}-}},\) \({\text{SiF}}_{{\text{6}}}^{{{\text{2}}-}},\) \({\text{ReO}}_{{\text{4}}}^{-};\) Br–, \({\text{NO}}_{{\text{3}}}^{-},\) \({{{\text{C}}}_{{\text{2}}}}{\text{N}}_{{\text{3}}}^{-};\) i = 1, 2; n = 0.5–2) in which the SCO is observed [18, 19]. In continuation of these works, we have synthesized and studied iron(II) complexes with L, containing cluster boron anions [B10H10]2– and [B12H12]2–. The salts of boron cluster anions are promising for the synthesis of biologically active complexes, including cytotoxic drugs [20–24].

Scheme 1. Structure of 2,6-bis(benzimidazol-2-yl)pyridine (L).
Previously, we synthesized iron(II) complexes with polynitrogen-containing ligands, in particular, with 1,2,4-triazoles and tris(pyrazol-1-yl)methanes, containing [B10H10]2– and [B12H12]2– as outer-sphere anions [8, 25–27]. Most of these compounds show spin crossover. It seemed expedient to continue research along this line. The search for new bi- and polyfunctional compounds is an important scientific challenge.
EXPERIMENTAL
For the synthesis, FeSO4 · 7H2O (Acros Organics), ascorbic acid medicinal, 2,6-bis(benzimidazol-2-yl)pyridine (Aldrich), and ethanol rectified were used. All reagents were used as purchased. The K2[B10H10] · 2H2O and K2[B12H12] complexes were synthesized as described elsewhere [28].
Synthesis of [FeL2][B10H10] · 2H2O (1) and [FeL2][B12H12] · H2O (2). A 0.14-g (0.5 mmol) sample of FeSO4 · 7H2O was dissolved in 6 mL of distilled water acidified with 0.12 g of ascorbic acid. To this solution, a 1.5-fold excess of K2[B10H10] · 2H2O (0.17 g, 0.75 mmol) or K2[B12H12] (0.17 g, 0.75 mmol) in 5 mL of water was added under stirring. Then, to each of the resulting solutions, a warm solution of 0.31 g (1 mmol) of L in 25 mL of ethanol was slowly added. Dark violet precipitates appeared immediately after mixing the solutions, which were stirred by a magnetic stirrer for 5 h. The precipitates were filtered off, washed two times with 1 mL of water and 1 mL of ethanol, and dried in air. The yield of compounds 1 and 2 was 95 and 85%, respectively.
For C38H40B10FeN10O2 (1) anal. calcd. (%): C, 54.8; H, 4.8; N, 16.8.
Found (%): C, 54.5; H, 4.4; N, 16.6.
For C38H40B12FeN10O (2) anal. calcd. (%): С, 54.4; H, 4.8; N, 16.7.
Found (%): C, 54.2; H, 5.0; N, 16.3.
C, H, N elemental analysis was carried out on a EuroVector EURO EA 3000 analyzer in the analytical laboratory of the Institute of Inorganic Chemistry, SB RAS.
X-ray diffraction analysis of fine crystalline compounds was performed on a Shimadzu XRD 7000 diffractometer (CuKα radiation, Ni filter, scintillation detector) at room temperature.
Static magnetic susceptibility of the samples was measured by the Faraday method in the temperature range 80–480 K. Temperature stabilization of the sample with an accuracy of 1 K during measurements was carried out using a Delta Electronics DTB9696 temperature controller. The heating and cooling rates of the samples were ~2–3 K/min. The external magnetic field strength of 7.3 kOe during the studies was maintained with a stabilization accuracy of ~2%. To study the dehydrated complexes, the samples under study were placed in open quartz ampoules and evacuated to a residual pressure in the measuring cell of 10–2 mm Hg, then an inert helium atmosphere at a pressure of 5 mm Hg was created. In the study of compounds containing crystallization water, the samples were sealed with atmospheric air in quartz ampoules. The effective magnetic moment was calculated by the formula μeff = \({{\left( {{\text{8}}\chi _{{\text{M}}}^{'}T} \right)}^{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0em} 2}}}},\) where \(\chi _{{\text{M}}}^{'}\) is the molar magnetic susceptibility corrected for the diamagnetic contribution according to the Pascal scheme. The temperatures of the direct (Тс↑) and reverse (Тс↓) transitions of the complexes under study were determined based on the condition d2μeff/dT2 = 0.
IR absorption spectra were recorded on Scimitar FTS 2000 (4000–400 cm–1) and Vertex 80 (400–100 cm–1) IR FT spectrophotometers. Samples were prepared as mineral and fluorinated oil mulls and polyethylene pellets.
Kubelka–Munk diffuse reflectance spectra (DRS) were recorded on a Shimadzu UV-3101 РС scanning spectrophotometer at room temperature.
Mössbauer spectra of the complexes were measured at 295 K on an NP-610 spectrometer with a 57Co (Rh) source.
FeK edge X-ray absorption spectra were collected in the standard transmission mode in the range 800 eV above the absorption edge (7112 eV) using a Si(111) double-crystal monochromator. The studies were performed on beamline 8 of the VEPP-3 storage ring, Siberian Synchrotron and Terahertz Radiation Center, Institute of Nuclear Physics, SB RAS. Measurements for [FeL2][B10H10] · 2H2O were carried out at two temperatures: T ~ 300 K (the compound in the low-spin (LS) state) and T = 450 K (the compound in the high-spin (HS) state). For [FeL2][B12H12] · H2O, the measurement was performed only at room temperature. When heated, the sample was placed in an furnace open at both ends; the temperature was controlled with a chromel–alumel thermocouple. The oscillating part (χ(k)) of the absorption spectrum was obtained with the VIPER software; the simulation was carried out with the EXCURV program for unfiltered (raw) spectra in the approximation of multiple scattering of the whole molecule without considering cluster boron anions. In modeling the structure of the ligand (L), in order to reduce the number of variable parameters, benzene rings were not taken into account, which, due to their structural distance from the central iron atom, have a weak effect on the shape of the EXAFS spectrum. The modeling procedure was carried out in the range of wave vectors Δk = 3.0–13 Å–1 with the weighting coefficient w = k3 and the amplitude suppression factor \(S_{0}^{2}\) = 1.0.
RESULTS AND DISCUSSION
The [FeL2][B10H10] · 2H2O and [FeL2][B12H12] · H2O complexes were isolated from a water/ethanol system at a Fe2+ concentration of ~0.15 mol/L and the stoichiometric Fe : L ratio. Ascorbic acid was used as a reducing agent and a weakly acidifying reagent. At the first stage, a solution of iron(II) closo-borate was prepared from an aqueous solution of FeSO4 with the use of a 1.5-fold excess of the K2[B10H10] · 2H2O or K2[B12H12] salt. At the second stage, these solutions were reacted with an ethanol solution of the ligand. The complexes were isolated in high yield (80–90%).
Table 1 presents key vibrational bands of L and complexes 1 and 2. The high-frequency range of the spectra of the complexes (3460–3630 cm–1) shows O–H stretching vibration bands. The spectrum of L shows in the range 3400–2800 cm–1 broad stretching vibration bands of NH groups involved in hydrogen bonds. In the spectra of the complexes, the ν(NH) bands become narrower and are observed at 3210 and 3183 cm–1 (1) and 3274 cm–1 (2). The high-frequency shift of the ν(NH) bands and their clearer manifestation in comparison with the L spectrum is probably associated with the weakening of hydrogen bonds on going from the ligand to the complexes. In the range 2500–2400 cm–1, the spectra show B–H stretching vibration bands. The position of the bands in the range of ring vibrations in the spectra of complexes 1 and 2 noticeably changes in comparison with that in the spectrum of L, which indicates the coordination of the nitrogen atoms of heterocycles to iron(II). This is also supported by the character of the spectra of 1 and 2 in the low-frequency range. There are bands at 280 cm–1 that are absent in the spectrum of the ligand, which can be attributed to the M–N stretching vibrations.
The DRS of complexes 1 and 2 in the visible and near-IR ranges show two broad absorption bands at 537 and 755 nm (1) and 510 and 709 nm (2). The bands in the 500–540 nm range with maxima at 537 (1) and 510 (2) cm–1 can be assigned to the d–d transition 1A1 → 1T1 in a strong distorted octahedral ligand field. The position of these bands is typical of low-spin octahedral iron(II) complexes with nitrogen-containing ligands with the FeN6 chromophore [4, 26]. The absorption bands in the range of 700–760 nm with maxima at 755 (1) and 709 nm (2) refer to the d–d transition 5Т2 → 5Е in high-spin octahedral Fe(II) complexes with nitrogen-containing ligands. For these forms of complexes, νHS = ΔHS. The splitting parameters for 1 and 2 have been calculated using the following approximations: νLS = ΔLS – C + 86B2/ΔLS, ΔHS ≈ 19B, and С = 4.41B [29–31] (Table 2). Comparison of the splitting parameters for the three classes of compounds studied by us—Fe(II) complexes with 1,2,4-triazoles [4], tris(pyrazol-1-yl)methanes [4], and 2,6-bis(benzimidazol-2-yl)pyridine ([19] and this work)—shows that the latter produces a stronger ligand field as compared to 1,2,4-triazoles, while the splitting parameters ΔLS and ΔHS for the complexes with tris(pyrazol-1-yl)methanes and 2,6-bis(benzimidazol-2-yl)pyridine are close to each other.
The Mössbauer spectra of the complexes (Fig. 1) are a superposition of lines arising from the HS and LS states of iron(II) atoms. The chemical shift δ (with respect to α-Fe) and the quadrupole splitting ε for each of the species have been extracted by processing the experimental spectra (Table 3). In both cases, the major species is the LS form with lower δ and ε values; the αHS value for the complex with the [B12H12]2– anion is almost twice as large as that for the complex with the [B10H10]2– anion.
The difference between the Mössbauer spectra of the iron(II) compounds synthesized in this work with the [B10H10]2– and [B12H12]2– anions from the previously studied complexes of the composition [FeL2]Ai (A = \({\text{SO}}_{{\text{4}}}^{{{\text{2}}-}},\) \({\text{SiF}}_{{\text{6}}}^{{{\text{2}}-}},\) \({\text{ReO}}_{{\text{4}}}^{-};\) Br–; \({\text{NO}}_{{\text{3}}}^{-};\) \({{{\text{C}}}_{{\text{2}}}}{\text{N}}_{{\text{3}}}^{-};\) i = 1, 2) [18, 19] is in the presence of lines of the HS iron(II) forms in the spectra at 295 K. The spectra of the previously studied complexes show the lines of the HS iron(II) species at room temperature only in one case. The δ and ε values for the LS forms of complexes 1 and 2 are in the same range of values as for the previously studied compounds. However, the trend in the behavior of these parameters (an increase in ε with an increase in δ), which we revealed earlier, is not observed for complexes with cluster boron anions. Noteworthy is one more feature of the Mössbauer spectra of complexes 1 and 2: the lines of the quadrupole doublet for the HS form of the complex with the [В10Н10]2– anion are narrow, and those for the complex with the [В12Н12]2– anion are broader. This suggests that the HS form generated upon spin crossover is well crystallized in the first case and is weakly crystallized state in the second case.
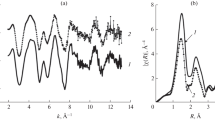
Figure 2 shows the FeK XANES spectra of [FeL2][B10H10] · 2H2O (solid line) and [FeL2][B12H12] · H2O (circles) measured at 300 K and [FeL2][B12H12] · H2O (dashed line) measured at 450 K. For the sample measured at T = 450 K and being in the high-spin state (dashed line), the absorption edge is displaced toward higher energies, which is evidence of an increase in the positive charge at the iron atom. Figures 3 and 4 show the Fourier transform magnitudes of experimental FeK EXAFS spectra (k2χ(k)) of complexes 1 and 2 not corrected. As follows from Fig. 3, the FT magnitudes for complexes 1 and 2 coincide within the margins of measurement error. This indicates that the structural parameters of the nearest environment of iron(II) ions are similar; therefore, the same model was used to simulate the EXAFS spectra for these two compounds. Heating [FeL2][B10H10] · 2H2O to 450 K (high-spin state) does not lead to radical changes in the structure of the environment of the iron atom (Fig. 4), no shifts of the maxima are observed, indicating no changes in the corresponding interatomic distances. One can only note a decrease in the amplitude of the maxima due to an increase in the temperature part of the Debye factor. Figure 5 and Table 4 show the structural data for the coordination core obtained in the course of simulation of the EXAFS spectrum of the [FeL2][B12H12] · H2O complex. The accuracy of determination of interatomic distances from EXAFS data is ±1% (for the nearest sphere of the environment). Figure 6 shows the Fourier transform magnitudes of the experimental (solid line) and simulated (dashed line) FeK EXAFS spectra (k2χ(k)) of the [FeL2][B12H12] · H2O complex. Measurements were performed at room temperature.
The results of studying the static magnetic susceptibility of the compounds under study are shown in Figs. 7 and 8. The complexes studied in the empirically selected temperature ranges of their stability demonstrate spin crossover both in the presence of water of crystallization and in the dehydrated state. The temperatures of the direct and reverse transitions are presented in Table 5.
In the high-spin state of the crystal water-containing complexes, μeff = 4.5–4.7 μB are achieved, which is somewhat lower than the theoretical value of 4.9 μB. In the low-spin form of these compounds, residual μeff = 0.5–1.0 μB are observed. Going from [FeL2][B10H10] · 2H2O to [FeL2][B12H12] · H2O is accompanied by a decrease in crossover temperatures by ~ 80 K and the disappearance of hysteresis loop (ΔTc) between the direct and reverse transitions.
Upon dehydration of the complexes, an increase is observed in the maximum transition temperature and the residual magnetic moment in the low-spin state to μeff = 1.2–1.6 μB. The tendency towards a decrease in the SCO temperature remains on going from [FeL2][B10H10] to [FeL2][B12H12]. For [FeL2][B10H10] in the temperature range of stability, μeff = 3.3 μB is achieved. With the removal of water molecules of crystallization, an increase/appearance of hysteresis between the direct and reverse transitions is also observed (Table 5).
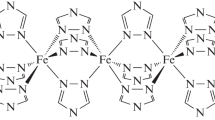
The results of the study of the obtained complexes and comparison with the literature [16, 17] and our earlier data [18, 19] show that the [FeL2][B10H10] · 2H2O and [FeL2][B12H12] · H2O complexes have an octahedral coordination polyhedron. Two ligands are coordinated to the iron(II) ion in a tridentate cyclic mode through two nitrogen atoms of the imidazole rings and the pyridine nitrogen atom to form the FeN6 coordination core.
CONCLUSIONS
New iron(II) closo-borate complexes with 2,6-bis(benzimidazole-2-yl)pyridine [FeL2][B10H10] · 2H2O and [FeL2][B12H12] · H2O have been synthesized. It has been demonstrated that both the initial compounds and their dehydrated analogues exhibit thermo-induced spin crossover 1А1 ⇔ 5Т2. The SCO temperatures significantly depend on the composition of the outer-sphere anion: the Тс values of [FeL2][B10H10] · 2H2O and [FeL2][B10H10] are significantly higher than Тс for [FeL2][B12H12] · H2O and [FeL2][B12H12].
REFERENCES
Spin Crossover in Transition Metal Compounds I–III, Ed. by P. Gütlich and H. Goodwin (Springer, 2004).
M. A. Halcrow, Spin-Crossover Materials Properties and Applications (Wiley, 2013).
O. Kahn, J. Kröber, and C. Jay, Adv. Mater. 4, 718 (1992).
L. G. Lavrenova and O. G. Shakirova, Eur. J. Inorg. Chem, 670 (2013).
M. Shatruk, H. Phan, B. A. Chrisostomo, and A. Suleimenova, Coord. Chem. Rev. 289–290, 62 (2015).
H. L. C. Feltham, A. S. Barltrop, and S. Brooker, Coord. Chem. Rev. 344, 26 (2017).
H. S. Scott, R. W. Staniland, and P. E. Kruger, Coord. Chem. Rev. 362, 24 (2018).
O. G. Shakirova, L. G. Lavrenova, A. S. Bogomyakov, et al., Russ. J. Inorg. Chem. 60, 786 (2015).
E. N. Frolova, T. A. Ivanova, O. A. Turanova, et al. Russ. J. Inorg. Chem. 63, 1012 (2018).
P. Gamez, J. S. Costa, M. Quesada, and G. Aromí, Dalton Trans., 7845 (2009).
S. Hayami, S. M. Holmes, and M. A. Halcrow, J. Mater. Chem. C 3, 7775 (2015).
M. Matsuda, H. Isozaki, and H. Tajima, Chem. Lett. 37, 374 (2008).
R. N. Muller, V. Elst, and S. Laurent, J. Am. Chem. Soc. 125, 8405 (2003).
J.-F. Letard, N. Daro, C. Aymonier, et al., Patent EP2391631 (2011).
A. Bousseksou, C. Vieu, J.-F. Letard, et al., EU Patent 1 430 552 (2004).
M. Boča, R. F. Jameson, and W. Linert, Coord. Chem. Rev. 255, 290 (2011).
R. Boča, P. Baran, M. Boča, et al., Inorg. Chim. Acta 278, 190 (1998).
L. G. Lavrenova, I. I. Dyukova, E. V. Korotaev, et al., Russ. J. Inorg. Chem. 65, 30 (2020).
A. D. Ivanova, E. V. Korotaev, V. Yu. Komarov, et al., New J. Chem. 44, 5834 (2020).
I. B. Sivaev, V. I. Bregadze, and N. T. Kuznetsov, Izv. Akad. Nauk, Ser. Khim., No. 8, 1256 (2002).
M. F. Hawthorne, Mol. Med. Today 4, 174 (1998).
Y. Zhu, Y. Lin, Y. Z. Zhu, et al., J. Nanomater. 2010, art. 409 320 (2010).
V. V. Avdeeva, E. A. Malinina, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 62, 1673 (2017).
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Coord. Chem. 46, 297 (2020).
O. G. Shakirova, L. G. Lavrenova, and V. N. Ikorskiy, et al., Chem. Sust. Dev. 10, 757 (2002).
M. B. Bushuev, L. G. Lavrenova, Yu. G. Shvedenkov, et al., Russ. J. Coord. Chem. 34, 190 (2008).
O. G. Shakirova, V. A. Daletskii, L. G. Lavrenova, et al., Russ. J. Inorg. Chem. 58, 650 (2013).
H. C. Miller and E. L. Muetterties, Inorg. Synth. 10, 81 (1967).
A. Lever, Inorganic Electronic Spectroscopy (Elsevier, Amsterdam, 1984; Mir, Moscow, 1987).
A. Hauser, Top Curr. Chem. 233, 49 (2004).
S. Sugano, Y. Tanabe, and H. Kamimura, Multiplets of Transition-Metal Ions in Crystals (Academic Press Pure and Applied Physics, New York, 1970).
ACKNOWLEDGMENTS
We are grateful to N.P. Korotkevich for recording the X‑ray powder diffraction patterns and to I.V. Yushina for recording the diffuse reflectance spectra.
Funding
The work was partially supported by the Russian Science Foundation (project no. 20-63-46026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Ivanova, A.D., Lavrenova, L.G., Korotaev, E.V. et al. High-Temperature Spin Crossover in Complexes of Iron(II) closo-Borates with 2,6-Bis(benzimidazol-2-yl)pyridine. Russ. J. Inorg. Chem. 65, 1687–1694 (2020). https://doi.org/10.1134/S0036023620110078
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620110078