Abstract
Interaction between the systems containing cobalt(II) or nickel(II) chlorides and 2-thiobarbituric acid (C4H4N2O2S, H2L) was examined by spectrophotometry and pH-metry at pH < 4, I = 0.1 (NaCl) and T = 20°C. The compositions and the overall stability constants (log β11) of protonated complex species were determined: [CoHL]+ (3.69 ± 0.04), [NiHL]+ (3.68 ± 0.08), and [M(HL)2]. The compositions of the Co(C4H3N2O2S)2 · 4H2O and Ni(C4H3N2O2S)2 · 5H2O salts isolated from the studied systems were confirmed by different physicochemical methods; their solubility constants (log KS) were calculated to be –12.82 ± 0.22 and –12.30 ± 0.10, respectively. A spectrophotometric technique was developed for determining thiobarbiturate ion in aqueous solutions. This technique is based on the absorbance of iron(III) thiobarbiturate complex [FeL]+ and can be recommended for analytical chemists and pharmacists.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Thiobarbituric acid (C4H4N2O2S, H2thioBar, H2L, 2-thiobarbituric acid, or malonylthiourea, a derivative of barbituric acid) is a parent compound giving rise to the thiobarbiturate class of drugs, which exhibit pronounced sedating, anesthetic, anticonvulsant, antisclerotic, and bacteriostatic properties. The best-known of them are sodium thiopental, thiobarbital, thiobutabarbital, thialbarbital, and methitural [1, 2]. Thiobarbituric acid is widely used for determining malonic dialdehyde [3], a product of lipid peroxidation, in experimental and clinical pharmacology. The researchers from the Laboratory of Biochemistry and Immunology of the Pitié-Salpêtrière Hospital (Paris, France) proposed a procedure for the colorimetric determination of phosphohexoisomerase with thiobarbituric acid to quantify the amount of fructose formed in enzyme-catalyzed processes [4]. Due to specific colorimetric reactions of H2thioBar, it can be used as an analytical reagent to detect p- and d-metal ions (Bi3+, Cu2+, Pd2+, Fe3+, Ru3+, etc.) [5]. Thiobarbiturate complexes of rare-earth elements are also used as precursors to synthesize the respective oxides, sulfides, oxysulfides, and oxysulfates [6–8], which are broadly employed in electrical engineering and materials science. In addition, the complexes containing thiobarbiturate anions as ligands possess biological and pharmacological properties. The tin(IV) complex was found to exhibit antibacterial and anticancer activities [9], while mixed-ligand salts of cobalt(II), nickel(II), and copper(II) with malonylthiourea and derivatives of arylidene-anthranilic acid possess antimicrobial activity [10].
Malonylthiourea can occur in a large number of keto-enol or lactam-lactim tautomeric forms. The isomeric forms are so diverse, since a thiobarbituric acid molecule contains three mobile hydrogen atoms (one atom in the methylene group >CH2 and two atoms in the imide groups >NH) and three potentially enolizable groups (two carbonyl >C=O and one thiocarbonyl group >C=S). However, equilibrium is preferentially attained between the thiocarbonyl-dicarbonyl (I) and thiocarbonyl-monocarbonyl (II) tautomers in aqueous solutions [5]:

Due to the presence of electron-donating nitrogen, oxygen, and sulfur atoms within the aforelisted functional groups, the acid can act as a mono- or bidentate ligand during complexation. The acidic properties, which are stronger than those of barbituric and violuric acids (for the first dissociation step) [11–13], are ensured by hydrogen atoms in the hydroxyl –OH (with allowance for the potential enolization of the >C=O group) and imide >NH groups [5, 14] (species I and II, positions 1 and 6).
Singh et al. [15] studied the thermodynamics of stepwise complexation of thiobarbituric acid with Cu2+, Fe2+, Zn2+, Co2+, and Ni2+ ions giving rise to the [ML]0 and [ML2]2– species by pH-metry at constant ionic strength I = 0.1 (NaClO4) at 18, 31 and 42°C. An analysis of the thermodynamic parameters (ΔG°, ΔH°, and ΔS°; 31°C) shows that enthalpy makes a major contribution in these reactions. The negative ΔG° values indicate that the reactions are spontaneous, while the relatively low ΔS° values along with further reasoning have led to a conclusion that in this case M2+ and H2thioBar act as soft electron acceptors and donors, respectively. This is indirectly confirmed by participation of sulfur, a “soft” electron-donating atom, in ligand coordination [5]. The logarithms of stepwise stability constants (æi) for Co2+ and Ni2+ (18°C) are 6.82 and 5.30; 6.22 and 4.45, respectively. Ahmadi et al. [16] reported on the overall stability constants (β2) of the complexes formed by Cu2+, Zn2+, Co2+, and Ni2+ ions with malonylthiourea, which were measured by spectrophotometric titration at pH 6, ionic strength of 0.2% w/v (NaCl), and temperature of 25°C. For Co2+ and Ni2+, the logarithms of the overall stability constants are 4.69 and 4.47, respectively. Nevertheless, Golovnev and Molokeev [5] attributed these constants to effective ones, since they do not take into account the possible protonation of the ligand. Data on composition of the complex species in the M2+–H2thioBar (M = Cu, Ni) systems at pH 3–10 were obtained by polarography, amperometric titration, and spectrophotometry [17]. It was found that (M : L) 1 : 1, 1 : 2, 1 : 6 and 2 : 1 complexes are formed for nickel(II) (the values of the stability constants were not presented). Polarographic studies detected no complexation between H2thioBar and Co2+, Zn2+, Cd2+, and Pb2+.
The vast majority of published papers are devoted to the synthesis, determination of the structure and study of the properties of complexes with malonylthiourea; it is noted that these compounds possess supramolecular structure. For instance, [Co(HL)2] was isolated from aqueous solution [18]; [Ni(H2L)2(HL)]Cl and [Co(H2L)2(HL)]Cl · H2O [19] along with [Co(H2O)2(H2L)4](CH3COO)2 · 2H2O [20] were isolated from ethanol solutions. The structures of inner-sphere complex salts of cobalt(II), nickel(II), iron(II), and cadmium with composition M(H2O)2(HL)2, which have a polymeric structure with octahedral coordination geometry of M2+ ion, were thoroughly described using the X-ray diffraction (XRD) and IR spectroscopy data in [5, 21]. HL– is coordinated via donor oxygen and sulfur atoms. The outer-sphere nickel(II) thiobarbiturate [Ni(H2O)6](HL)2 · 2H2O is also known; right after being removed from the solution, it is rapidly converted to an inner-sphere complex salt with the composition specified above [21].
The aim of the present study is to determine the conditions under which protonated complex species are formed between 2-thiobarbituric acid and Co2+ (d7, 0.089 nm) or Ni2+ cations (d8, 0.083 nm) in an aqueous solution, to determine their compositions and stability constants, to isolate salts from the respective systems and perform their physicochemical analysis. These study objects were selected due to the biological importance of both the cations (biometals belonging to essential micronutrient elements) and thiobarbituric acid, which is an exogenous bioligand. Furthermore, the electrostatic parameters of these ions are close, so they form compounds with similar chemical properties.
EXPERIMENTAL
Reagents and equipment. 2-Thiobarbituric acid of pure for analysis grade (manufactured in Russia) and cobalt(II) and nickel(II) chloride hexahydrates of chemically pure grade were used as initial reagents without additional purification. Constant ionic strength I = 0.1 (ensured by sodium chloride of chemically pure grade) and temperature of 20°C were maintained in the working solutions. Potentiometric titration with an alkali solution was used to refine acid concentration; concentrations of respective metal salts were refined by direct trilonometric titration using the known procedures [22].
Spectrophotometric measurements in the UV and visible regions were conducted on a PE-5400UV spectrophotometer (Ecros) in 10-mm thick quartz cells. pH was measured using a pH meter 673 in a concentration scale; for this purpose, a glass electrode was pre-calibrated with respect to (H,Na)Cl solutions having different concentrations of hydrochloric acid at a constant ionic strength I = 0.1. Simultaneous thermal analysis (STA) of malonylthiourea and its salts was conducted on a NETZSCH STA 449 F1 instrument in an air flow. A typical measurement procedure included a non-isothermal heating from 30 to 600°C at a rate of 10°C/min in precalcined alumina crucibles. Sample weight was ~ 5 mg. The transmittance IR spectra over the range 4000–400 cm–1 were recorded for solid samples in KBr pellets on a Nicolet 6700 FT-IR spectrometer with a diamond accessory. The solubility constants (KS) of the synthesized salts and the stability constants of the complexes (β11) were calculated with allowance for a possible protonation of ligand anions and first-step hydrolysis of metal cations using the procedures described in [23]. The detailed procedures of experiments and calculations were presented in [24].
Determination of the composition and stability of complex species. Spectrophotometric method and potentiometric titration of thiobarbituric acid with sodium hydroxide solution made it possible to determine the protonation constant (I = 0.1, 20°C) of its single-charged anion (æ2): log æ2 = 2.37 ± 0.06 and log æ2 = 2.30 ± 0.01, respectively [14]. Further calculations were conducted using the value determined from the pH-metric data. The overall protonation constants of the double-charged thiobarbiturate anion were taken from [15] for 18°С (log B1 = 10.55; log B2 = 12.85). The temperature difference was not taken into account, since it was negligible. The B2 value involves the value found experimentally æ2 (log B2 = log B1 + log æ2).
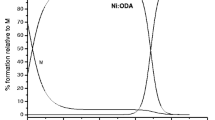
The compositions of complex species formed in the binary systems under study were identified spectrophotometrically using the molar-ratio method, as well as by analyzing the results of calculations using the pH-metric data. The dependence of absorbance А on the cL/cM ratio (Fig. 1, the saturation curve in the Ni2+–H2thioBar system) demonstrates that the solution (pH 2.7) contains complexes with 1 : 2 composition: [M(HthioBar)2]0 (identically to the Co2+–H2thioBar system). It is most likely that the inner coordination sphere of these complexes contains protonated ligand species, since the distribution diagram of equilibrium acid species as a function of pH (Fig. 2) indicates that HthioBarˉ is the dominant species at a given pH.
Changes in absorbance ΔA (ΔA = Acomplex – AM) as a function of the \({{{{C}_{{\text{L}}}}} \mathord{\left/ {\vphantom {{{{C}_{{\text{L}}}}} {{{C}_{{{\text{N}}{{{\text{i}}}^{{2 + }}}}}}}}} \right. \kern-0em} {{{C}_{{{\text{N}}{{{\text{i}}}^{{2 + }}}}}}}}\) ratio (λeff = 395 nm, pH 2.7, I = 0.1 (NaCl), T = 20°C).
Distribution diagram of equilibrium malonylthiourea species depending on pH of the aqueous solution: (α2) H2thioBar, (α1) HthioBar–, and (α0) thioBar2– (cL = 0.01 М; log B1 = 10.55; log B2 = 12.85; “ Yield of the Acid” software [23]).
The overall stability constants of the thiobarbiturate complexes were determined by pH-metric titration of a mixture of cobalt(II) or nickel(II) salt (with the known free acidity) and thiobarbituric acid with a solution of prestandardized carbonate-free alkali containing the background electrolyte under bubbling of purified nitrogen gas. The stability constants of protonated complexes [MHthioBar]+ were calculated at pH 2.3–3.3 according to the pH-metric data using the Bjerrum 1 software [23]. The β11 values expressed in logarithmic form for cobalt(II) and nickel(II) are 3.69 ± 0.04 and 3.68 ± 0.08, respectively. The required experimental data and the calculated logβ11 values are presented in Tables 1 and 2.
Synthesis and analysis of the isolated Co(C4H3N2O2S)2 · 4H2O and Ni(C4H3N2O2S)2 · 5H2O salts. Cobalt(II) and nickel(II) thiobarbiturates were synthesized at pH 3.5–4.0 by interaction between the cooled down saturated aqueous solutions of the respective metal chlorides and malonylthiourea, whose solution was partially neutralized by sodium hydroxide, at a M2+ : H2thioBar : NaOH (1 : 2 : 1.2) molar ratio. The precipitated crystals were filtered off, washed with ethanol, and dried to constant weight in a desiccator cabinet at 45°C.
The isolated thiobarbiturates were pink and yellow fine-grained substances. Metal contents in the synthesized salts were determined gravimetrically according to the weights of the respective oxides Co3O4 and NiO obtained after the samples had been calcined in a muffle furnace at 800°C for 3 h and by direct trilonometric titration [22] (with the salts being pre-dissolved in (H, Na)Cl solutions). Trilon B concentration was determined using the solutions with the known concentration of metal(II) in the presence of thiobarbituric acid. The content of thiobarbiturate ion in salts was determined using a spectrophotometric technique developed by us; this technique is similar to the one reported earlier for the barbiturate ion [11] and is based on absorbance of the thiobarbiturate complex of iron(III) [FethioBar]+ (λeff = 490 or 540 nm, \({{C}_{{{\text{F}}{{{\text{e}}}^{{3 + }}}}}}\) = 0.01 М, cHCl = 0.1 М, Vtotal = 25.0 mL, pH 2.3) under the conditions close to its maximum yield (~ 76%, Fig. 3). The Beer–Lambert law is fulfilled within the range of thiobarbiturate anion concentrations of (0.5–2.5) × 10–3 М. The resulting calibration curves at 490 and 540 nm are characterized by good coefficients of determination (0.9885 and 0.9919, respectively). The least squares fitting of these curves (tα = 2.57) yields the following parameters of straight-line equation:
(in this case, coefficient a is non-significant, so we assume it to be zero):
b = (130.0 ± 18.0) at λeff = 490 nm;b = (100.9 ± 12.9) at λeff = 540 nm.
Distribution diagram of Fe3+ (α0) and FethioBar+ (α1) species depending on pH of the aqueous solution (cL = 0.01 M; log β1 = 11.05; log B1 = 10.55; log B2 = 12.85; “Yield of the complex” software [23]).
The composition and stability of the [FethioBar]+ complex were determined preliminarily by spectrophotometry and potentiometry.
The contents of crystallization water and metal (Co3O4 and NiO) were estimated from the thermogravimetric analysis data. The results obtained by chemical analysis of cobalt(II) and nickel(II) thiobarbiturates are presented below (the values averaged over the gravimetric, thermogravimetric, and titrimetric data are reported for metal contents).
For Co(C4H3N2O2S)2 · 4H2O anal. calcd. (%): Co, 14.12; C4H3N2O2S–, 68.61; H2O, 17.27.
Found (%): Co, 14.74; C4H3N2O2S–, 65.13; H2O, 19.59.
For Ni(C4H3N2O2S)2 · 5H2O anal. calcd. (%): Ni, 13.49; C4H3N2O2S, 65.81; H2O, 20.70.
Found (%): Ni, 13.53; C4H3N2O2S–, 62.70; H2O, 18.62.
The presence of crystallization water in salts was also qualitatively confirmed by IR spectra; the frequencies of selected bands are presented below:
IR spectrum of Co(C4H3N2O2S)2 · 4H2O (ν, cm–1): 3353 w, 3106 w, 3055 w, 2662 w, 1585 vs, 1551vs, 1474 w, 1392 vs, 1287 vs, 1230 s, 1190 vs, 1010 s, 944 m, 886 vs, 811vs, 731 w, 697 m, 627 m, 606 s, 538 vs, 488 vs, 414 w.
IR spectrum of Ni(C4H3N2O2S)2 · 5H2O (ν, cm–1): 3392 w, 3104 w, 3065 w, 2890 w, 2668 w, 1605 vs, 1540 vs, 1443 w, 1406 s, 1295 s, 1250 w, 1203 vs, 967 w, 869 w, 814 m, 792 vs, 632 m, 604 m, 526 vs, 471 w, 416 w.
Determination of solubility of the synthesized salts. The solubility constants of the synthesized salts KS = [M2+][HthioBarˉ]2 were determined at pH 2.5–5.3 for Co(C4H3N2O2S)2 · 4H2O and pH 2.5–2.8 for Ni(C4H3N2O2S)2 · 5H2O at 20°C. Small samples of cobalt(II) and nickel(II) thiobarbiturates were placed into 0.1 M (H, Na)Cl solutions. The suspensions were periodically shaken in vessels with ground-glass stoppers and allowed to stand for several days until a heterogeneous equilibrium (constant pH) was attained. The saturated solutions were filtered; their pH was measured; concentrations of M2+ ions were determined by trilonometric titration. The equilibria attained in saturated aqueous solutions of salts without allowance for complexation and the respective constants can be written as:
KS = [M2+][HthioBar–]2;
æ2 = [H2thioBar]/([HthioBar–][H+]);
Kh1 = ([MOH+][H+])/[M2+].
One can see from Eq. (1) that cM = csalt (csalt is the solubility of salt, mol/L) in a saturated solution. Then, the material-balance equation with respect to metal ion will be written as:
The value [MOH+] = [M2+]Kh1/[H+] was found from Eq. (3) for Kh1; ω = 1 + Kh1/[H+] is a function of hydrolysis; Kh1 is the constant for the first-step hydrolysis of M2+ ions. From Eq. (4) we obtain that [M2+] = csalt/ω. With allowance for equilibrium (1), we obtain from the material-balance equation (5):
where f = 1 + æ2[H+] is the function of single-charged thiobarbiturate anion protonation (æ2 is the protonation constant of HthioBar– ions), that [HthioBar–] = 2csalt/f. The value [H2thioBar] = [HthioBar–][H+]æ2 was found from Eq. (2) for æ2. By substituting the respective values into the expression KS = [M2+][HthioBar–]2, we obtain that:
Table 3 summarizes the experimentally determined solubilities of cobalt(II) and nickel(II) thiobarbiturates in 0.1 M (H, Na)Cl solutions and their calculated solubility constants without allowance for complexation. Calculations of the solubility constants of salts with allowance for possible complexation using the “Solubility” software [23] yields the following data: pKS = 12.82 ± 0.22 for Co(C4H3N2O2S)2 · 4H2O and pKS = 12.30 ± 0.10 for Ni(C4H3N2O2S)2 · 5H2O.
RESULTS AND DISCUSSION
For the complexes formed by divalent metals M2+ with H2thioBar, [MthioBar]0 and [M(thioBar)2]2‾ are the predominant species in aqueous solutions at pH > 6 [15, 16]. Protonated complex species are generally typical of nitrogen-containing ligands [25]. Therefore, one should expect that various protonated thiobarbiturates will be formed in the acidic region, since because of the electrostatic characteristics of Co2+ and Ni2+ ions they cannot replace the second proton from the single-charged thiobarbiturate anion (pKa2 = 10.55), which is the predominant species within a broad pH range.
An analysis of saturation curves (Fig. 1) in the M2+–H2thioBar systems shows that 1 : 2 complexes are formed at pH 2.7, thus indicating that coordination the deprotonated ligand species does not take place at this pH. The resulting titration curves (Fig. 4) of thiobarbituric acid solutions and its mixtures containing pre-acidified (cHCl = 0.01 M) cobalt(II) or nickel(II) chlorides with an alkali have different shapes, thus demonstrating that cations of these metals replace the proton and providing indirect evidence for complexation processes occurring in the systems under study. The experimentally determined equivalent volumes of alkali (Vexp), which amount to 6.53 (Co2+) and 6.41 mL (Ni2+), agree fairly well with the theoretically expected value (Vtheor = 6.50 mL) calculated with allowance for neutralization of one proton released during the interaction. According to the species distribution diagram (Fig. 2), the yield of acid species within the pH range 2.3–3.3 varies from 46.6 to 92.0% for HthioBar– and from 53.4 to 8.0% for H2thioBar. With allowance for this fact, the formation of protonated thiobarbiturate complexes of cobalt(II) and nickel(II) can be represented by ionic equilibria:
The almost identical positions of the titration curves of the M2+–H2thioBar systems due to the similar electrostatic characteristics of the ions under study suggest that the resulting complexes have similar stability, which is also evidenced by their stability constants (log β11) calculated using the pH-metric data. The stability constants for [CoHthioBar]+ and [NiHthioBar]+ are 3.69 ± 0.04 and 3.68 ± 0.08, respectively. Overall, the proposed interaction model is experimentally confirmed by the titration curves, the spectrophotometric data, and consistency achieved in determining the stability constants. The calculations using the “Bjerrum 2” software [23] show that the simultaneous dominance of [MHthioBar]+ and [M(HthioBar)2]0 complex species in the solution is not observed at pH 2.3–3.3, since the condition cL > cM is not met. Therefore, the presence of biligand protonated species was not taken into account when calculating the β11 values.
The yield diagram of acid species (Fig. 2) shows that HthioBar– anions are dominant (94.1–98.3%) in the range of pH 3.5–4.0 where cobalt(II) and nickel(II) salts were synthesized. Since proton of the ‒OH group is the first one to dissociate within an acid molecule (pKa1 = 2.30), O-coordination should be expected. The imide group is deprotonated in the strongly alkaline medium (pKa2 = 10.55). Therefore, N-coordination can be ruled out because the nitrogen atom in the –NH– group in HthioBar– is blocked by a proton. The ions under study cannot replace the second proton, so thiobarbiturates containing the protonated ligand species are precipitated from saturated solutions. According to the IR spectroscopy data and studies [5, 21], O,S-coordination is observed in the synthesized Co(C4H3N2O2S)2 · 4H2O and Ni(C4H3N2O2S)2 · 5H2O salts. The spectra of the analyzed compounds contain low-intensity bands at 3400–3300 cm–1 corresponding to ν(O–H) vibrations of bound water molecules. The characteristic bands ν(>C=O) are shifted towards lower frequencies compared to those in H2thioBar (1720–1640 cm–1), thus confirming that oxygen atoms are involved in ligand coordination. The disappearance of the peak at 1165–1145 cm–1 ascribed to ν(>C=S) is an indication of S-coordination [5], which also agrees with the presence of low-intensity bands at 1551 and 1540 cm–1 for the cobalt(II) and nickel(II) salts, respectively, whose stretching vibration frequencies are lower than those observed for the acid (at 1561 cm–1). These signals are ascribed to the –NH–(–C=S) cyclic thioamide structure and demonstrate that the coordinated ligand exists in the thiocarbonyl form. The minor differences in the region where ν(>N–H) vibrations are detected (~10 cm–1) are caused by the involvement of these groups in hydrogen bonding. According to the STA data, two water molecules are cleaved in salts in the high-temperature region (T > 200°C), which may indicate that they were located in the inner sphere (at lower temperatures cobalt(II) thiobarbiturate tetrahydrate and nickel(II) thiobarbiturate pentahydrate lose two and three water molecules, respectively). The coincidence between the main frequencies in the IR spectra, as well as the similar intervals of thermal destruction for the salts synthesized in this study, and the data reported in [5, 21] give grounds for claiming that the complexes have the same composition.
Zaki and Mohamed [20] showed that the spectra of zinc and cadmium complex salts of similar composition feature no bands corresponding to ν(>N–H) vibrations (3200–3000 cm–1), which may indicate that the nitrogen atom in the imide group binds to M2+ ions or the ligand undergoes tautomeric conversion to the enol-thiol form prior to coordination. The latter variant is the most plausible one, since the researchers believe that tautomerization of the keto form of the acid to the enol-thiol form is a prerequisite for ligand binding. In general, the O,S-coordination was also proposed in [20]. Weak vibrations at 430–410 cm–1 corresponding to ν(M–S) indicate that the M–S bond is present. Signals are also observed in this region for the salts synthesized in the current study: at 414 cm–1 for the cobalt(II) salt and at 416 cm–1 for the nickel(II) salt. It is clear that the bond between the metal and the acid residue in the synthesized thiobarbiturates is substantially covalent due to the polarizing effect of d‑metal cations on the one hand and, on the other hand, high polarizability of the acid residue as verified by the low values of their KS: 5.40 × 10–9 and 2.63 × 10–8 (without allowance for complexation); 1.51 × 10–13 and 5.01 × 10–13 (with allowance for complexation) for cobalt(II) and nickel(II) salts, respectively. Furthermore, these structures are additionally stabilized by intra- and intermolecular hydrogen bonds (N−H···O and O−H···O), as well as π–π interactions between the HthioBar– ligands [5, 21], which are also responsible for their low solubility.
REFERENCES
M. D. Mashkovskii, Mediaments Novaya Volna, Moscow, 2012) [in Russian].
R. M. C. Dowson, D. C. Elliot, W. H. Elliot, and K. M. Jones, in Data for Biochemical Research (Clarendon, Oxford, 1986; Mir, Moscow, 1991).
K. M. Oldham and P. E. Bowen, J. Am. Diet. Assoc. 98, 1001 (1998). https://doi.org/10.1016/S0002-8223(98)00230-2
A. German, A. Galli, C. Maison, et al., Clin. Chim. Acta 22, 551 (1968). https://doi.org/10.1016/0009-8981(68)90103-4
N. N. Golovnev and M. S. Molokeev, 2-Thiobarbituric Acid and Its Metal Complexes: Synthesis, Structure, and Properties (Siberian Federal Univ., Krasnoyarsk, 2014) [in Russian].
N. N. Golovnev and M. S. Molokeev, Russ. J. Coord. Chem. 40, 648 (2014). https://doi.org/10.7868/S0132344X14090035
N. N. Golovnev, M. S. Molokeev, S. N. Vereshchagin, et al., J. Coord. Chem. 68, 1865 (2015). https://doi.org/10.1080/00958972.2015.1031119
N. N. Golovnev, M. S. Molokeev, I. V. Sterkhova, et al., Russ. J. Stuct. Chem. 58, 539 (2017). https://doi.org/10.15372/JSC20170315
V. I. Balas, I. I. Verginadis, G. D. Geromichalos, et al., Eur. J. Med. Chem. 46, 2835 (2011). https://doi.org/10.1016/j.ejmech.2011.04.005
M. A. El-Gahami, S. A. Ibrahim, and H. M. A. Salman, Synth. React. Inorg. Met.-Org. Chem. 21, 1497 (1991). https://doi.org/10.1080/15533179108020625
N. M. Korotchenko and N. A. Skorik, Russ. J. Inorg. Chem. 45, 1099 (2000).
N. M. Korotchenko and N. A. Skorik, Russ. J. Inorg. Chem. 47, 790 (2002).
N. M. Korotchenko and N. A. Skorik, Russ. J. Inorg. Chem. 57, 133 (2012). https://doi.org/10.1134/S0036023612010135
A. P. Lakeev, N. M. Korotchenko, and E. R. Sayfulin, J. Sib. Fed. Univ. Chem. 12, 6 (2019). https://doi.org/10.17516/1998-2836-0104
B. R. Singh, R. K. Jain, M. K. Jain, et al., Thermochim. Acta 78, 175 (1984). https://doi.org/10.1016/0040-6031(84)87144-0
F. Ahmadi, A. H. M. Sarrafi, and M. M. Ghashghaee, Eur. J. Chem. 6, 47 (2009). https://doi.org/10.1155/2009/965619
R. J. Murphy and G. Svehla, Anal. Chim. Acta 99, 115 (1978). https://doi.org/10.1016/S0003-2670(01)84503-7
K. S. Siddiqi, P. Khan, S. Khan, et al., Synth. React. Inorg. Met.-Org. Chem. 12, 681 (1982). https://doi.org/10.1080/00945718208082685
M. S. Masoud, A. M. Heiba, and F. M. Ashmawy, Trans. Met. Chem. 8, 124 (1983). https://doi.org/10.1007/BF01036097
Z. M. Zaki and G. G. Mohamed, Spectrochim. Acta A 56, 1245 (2000). https://doi.org/10.1016/S1386-1425(99)00225-5
N. N. Golovnev, M. S. Molokeev, S. N. Vereshchagin, et al., Polyhedron 70, 71 (2014). https://doi.org/10.1016/j.poly.2013.12.021
G. Schwarzenbach and H. Flaschka, Die komplexometrische Titration (Ferdinand Enke, Stuttgart, 1965; Khimiya, Moscow, 1970).
N. A. Skorik and E. B. Chernov, Personal Computers in the Chemistry of Complex Compounds (Tomsk Univ. Press, Tomsk Univ., 2009) [in Russian].
V. N. Kumok and N. A. Skorik, Laboratory Work in the Chemistry of Complex Compounds (Tomsk Univ. Press, Tomsk Univ., 1983) [in Russian].
R. M. Smith and A. E. Martell, Critical Stability Constants (Plenum, New York/London, 1976), Vol. 2.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Terpilovskaya
Rights and permissions
About this article
Cite this article
Lakeev, A.P., Korotchenko, N.M. Cobalt(II) and Nickel(II) Compounds with 2-Thiobarbituric Acid. Russ. J. Inorg. Chem. 65, 1232–1239 (2020). https://doi.org/10.1134/S0036023620080082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620080082