Abstract
Nanocrystalline powders of lead sulfide with particle size from 5 to 105 nm have been synthesized by chemical deposition from aqueous solutions of lead acetate or nitrate using sodium sulfide as sulfidizing agent and in the presence of sodium citrate or Trilon B as complexing agents. The exposure of the nanopowders to air for six years has shown that PbS nanopowders obtained in the presence of sodium citrate Na3Cit, which behaves as both complexing and stabilizing agent, display the largest stability of phase composition. The stabilizing role of Na3Cit is due to its ability to form a shell on nanoparticle surface to prevent lead sulfide oxidation. It has been established that nanoparticle size remains constant and stable upon long-term keeping in air. The phase composition of PbS nanopowders prepared using Trilon B gradually changes owing to oxidation into lead sulfate upon long-term exposure to air.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Under common conditions, bulk lead sulfide PbS is a narrow-band semiconductor with cubic (space group \(Fm\overline 3 m\)) structure B1 [1] and band gap width of ~0.41 eV [2, 3]. The electronic properties of nanocrystalline PbS [3, 4], like the properties of other sulfide semiconductors, considerably differ from those of macrocrystalline analogs [5]. This fact caused increased interest in nanosized lead sulfide because PbS nanofilms and nanopowders can be used to extend the spectral range of infrared (IR) detectors, in lasers of near IR radiation, and solar cells. Nanocrystalline lead sulfide can be used in superfast optical switches and routers [6–8]. The most common devices based on macrocrystalline PbS are fire detectors, thermal source systems (night viewing devices) [8–11], therefore, the stability of size and phase composition of PbS nanoparticles in time is very important for the possible application of nanocrystalline PbS in such devices. Along with structural and phase transformations [12, 13], nanocrystalline lead sulfide undergoes oxidation and recrystallization [3, 4, 14]. Therefore, the wide application of nanocrystalline lead sulfide in equipment and devices requires to know the stability of size and phase composition of PbS nanoparticles on long-term use in air under common conditions. There is no this information in the literature.
In this work, we studied for the first time the effect of long-term (up to 6 years and longer) exposure to air on the phase composition and size of lead sulfide nanoparticles prepared from lead acetate or nitrate in the presence of sodium citrate Na3Cit or Trilon B as complexing agents.
EXPERIMENTAL
Nanocrystalline powders of lead sulfide PbS were obtained by chemical deposition from aqueous solutions of lead salts and sulfidizing agent. Lead acetate Pb(CH3COO)2 (Pb(AcO)2) or nitrate Pb(NO3)2 were used as a source of lead ions Pb2+, while sodium sulfide Na2S was used as a source of sulfide ions S2–. Sodium citrate Na3C6H5O7 (Na3Cit) or disodium ethylenediaminetetraacetate Na2H2-EDTA (C10H18N2Na2O10, Trilon B) were used as complexing and stabilizing agents. The synthesis using Trilon B was carried out with addition or in the absence of acetic acid (CH3COOH). All solutions were prepared from bidistilled water with pH 6.7–6.9. The synthesis of PbS nanoparticles was conducted at 298 K, pH of reaction mixtures was from 3.00 to 6.25. The pH value was monitored with a Hanna Instruments™ HI73127 pH meter.
Lead sulfide deposition was carried out according to the reaction schemes:
The synthesis was conducted in the following order: complexing agent was added to lead acetate or nitrate, next, the mixture was diluted to 100 mL, and the resultant solution was mixed with 100 mL of Na2S solution.
Due to low value of solubility product Ksp for lead sulfide PbS (at 298 K Ksp = 2.5 × 10–27 [15] or 8.0 × 10–28 [16]) the formation of PbS at sufficient content of Na2S in reaction mixture proceeds very fast (over 1–2 s). As a result, reaction mixture first becomes black and next PbS particles deposite over several minutes and solution becomes transparent. Synthesis duration to provide lead sulfide deposition is 5 min. Immediately after deposition, the resultant PbS deposite was triply washed with distilled water by decantation, separated by filtration, and dried in air at 323 K.
The phase composition and average particle size of nanopowders was determined by X-ray diffraction [17, 18]. X-ray measurements were performed on a Shimadzu XRD-7000 diffractometer using the Bragg–Brentano geometry and CuKα1.2 radiation in 2θ angular range from 18° to 90° with a step size of Δ(2θ) = 0.03° and step time of 10 s. X-ray diffraction patterns were numerically analyzed using HighScore Plus software package [19]. Diffraction reflections were described by pseudo Voigt function. The broadening of diffraction reflection β(2θ) was determined as β(2θ) = [(FWHMexp)2 – (FWHMR)2]1/2, where FWHMexp is the full width of experimental diffraction reflection at the half height, FWHMR is the instrumental function of angle resolution of diffractometer. Resolution function FWHMR(2θ) = \({{(u{{{{\tan}}}^{2}}{{\theta }} + v{{\tan\theta }} + w)}^{{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-0em} 2}}}}\) for the Shimadzu XRD-7000 diffractometer was determined in a special diffraction experiment on a standard sample of cubic lanthanum hexaboride LaB6 (NIST Standard Reference Powder 660a).
The average size D of particles (more strictly, average size of coherent scattering domains) of prepared lead sulfide powders and the same powders after keeping (storage) in air was assessed by the broadening of diffraction reflections from the dependence of reduced broadening β*(2θ) = [β(2θ)cos θ]/λ of reflections on scattering vector s = (2 sin θ)/λ. Let us note that allowance made for instrumental function FWHMR is important in the study of nanoparticles larger than 40 nm, for which FWHMexp value is relatively low. For nanoparticles smaller than 30 nm, FWHMexp\( \gg \) FWHMR, therefore allowance for instrumental error is within the size determination error D.
Lead sulfide nanopowders were stored in a Sanplatec MB desiccator under residual air pressure of 0.2 atm (2 × 104 Pa) to prevent moisture adsorption. The full shelf life was up to 6 years with occasional control of phase composition and nanoparticle size.
The phase composition of the prepared PbS nanopowders after keeping for ~1000–1300 and ~2000–2300 days was determined from X-ray diffraction data using Match! ©Crystal Impact software package [20]. The microstructure and elemental chemical composition of PbS powders were studied by scanning electron microscopy using a JEOL-JSM LA 6390 microscope with a JED 2300 Energy Dispersive X-ray Analyzer. To study PbS nanoparticles, we also used a JEOL JEM-2010 transmission electron microscope.
RESULTS AND DISCUSSION
Reagent concentrations in reaction mixtures used for the synthesis of lead sulfide nanopowders and the size of PbS nanoparticles in the powders are presented in Table 1.
According to X-ray diffraction data, all nanocrystalline powders obtained by the deposition of colloidal PbS particles have cubic (space group \(Fm\overline 3 m\)) lattice of type B1 with period aB1 from 0.5934 to 0.5938 nm (Table 1).
According to the results of energy dispersive X-ray analysis (EDX), the content of Pb, S, and O in the prepared dried monophasic powders of lead sulfide is 86 ± 2, 13 ± 1, and 1.0 ± 0.5 wt % (Fig. 1). According to EDX data, admixture oxygen is distributed over the surface of agglomerated nanoparticles. Water was removed from the nanopowders upon annealing; therefore, we can suppose that the main part of admixture oxygen belongs to adsorbed moisture, while remaining oxygen is in chemosorbed state. The synthesis was carried out in acidic reaction mixtures at pH < 6.25. The formation of lead hydroxo complexes under these experimental conditions is negligible; they appear in alkaline media at pH > 9 [3, 4].
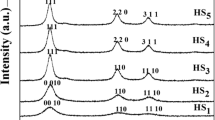
The synthesis of lead sulfide nanopowders 1–4 from aqueous solutions of lead acetate Pb(OAc)2 or nitrate Pb(NO3)2 with sodium sulfide Na2S in the presence of sodium citrate Na3Cit as a complexing agent allowed preparation of PbS nanoparticles with size from 5 to 7 nm. Figure 2 shows the typical X-ray diffraction patterns of PbS nanopowders obtained from reaction mixtures 1 and 3 containing Pb(AcO)2 or Pb(NO3)2, Na2S, and Na3Cit, and the same nanopowders after storage for 1010–1300 and 2030–2325 days.
Effect of exposure to air on the phase composition of nanopowders deposited from reaction mixtures 1 and 3 (Table 1) with sodium citrate: (a): (1) nanopowder 1, D = 5 ± 1 nm; (2) exposure to air for 1300 days, 100 wt % PbS; (3) exposure to air for 2325 days, 100 wt % PbS. (b): (4) nanopowder 3, D = 7 nm; (5) exposure to air for 1010 days, 95 wt % PbS + 5 wt % PbSO4; (6) exposure to air for 2030 days, 82 wt % PbS + 18 wt % PbSO4.
The average size D of PbS nanoparticles deposited from reaction mixture 1 with Pb(AcO)2, Na2S, and Na3Cit concentrations of 50, 50, and 25 mmol/L is 5–6 nm. The replacement of lead acetate Pb(OAc)2 by nitrate Pb(NO3)2 in reaction mixture 3 allowed preparation of PbS nanopowders with average particle size of ∼7 nm (Table 1). Let us note that change in Na3Cit concentration in reaction mixtures 1–4 from 25 to 12.5 mmol/L did not lead to change in the X-ray diffraction patterns and phase composition of PbS nanoparticles.
The X-ray diffraction patterns of powder 1 (Fig. 2a) deposited from lead acetate, sodium sulfide, and sodium citrate solutions registered after its exposure to air for 1300 and 2325 days still contained only diffraction reflections of cubic (space group \(Fm\overline 3 m\)) lead sulfide PbS, no change in the phase composition and size of nanoparticles took place.
The X-ray diffraction patterns of nanopowder 3 (Fig. 2b) deposited from lead nitrate, sodium sulfide, and sodium citrate solutions after storage for 1010 days showed weak diffraction reflections corresponding to orthorhombic (space group Pnma) lead sulfate PbSO4 along with reflections of cubic lead sulfide. Thus, nanopowder 3 showed emergence of PbSO4 particles, lead sulfate content is ~5 wt %. After 2030-day storage, this nanopowder contained ~18 wt % PbSO4. We detected no particles of mixed composition.
Nanopowders 1 and 2 prepared from lead acetate, sodium sulfide, and Na3Cit after more than six year storage (2325 days) retained size and phase composition of PbS nanoparticles. This fact seems to be due to formation of protective carbon-containing shell that prevents oxidation of the surface of PbS nanoparticles. In the synthesis from lead nitrate (nanopowders 3 and 4), the content of sodium citrate in reaction mixtures proved to be insufficient to form continuous citrate shell on nanoparticles, therefore, their gradual oxidation began upon long-term storage.
Sodium citrate in the synthesis of lead sulfide behaves as complexing and stabilizing agent that prevents nanoparticle growth. Citrate ion \({\text{C}}{}_{{\text{6}}}{{{\text{H}}}_{{\text{5}}}}{\text{O}}_{{\text{7}}}^{{{\text{3}} - }}\) contains three negatively charged oxygen ions O–. The introduction of citrate in aqueous solution with sulfide particles, citrate ions fix on particle surface via one of O– ions, whereas two other negatively charged ions are directed to solution [21, 22]. As a result, a negatively charged citrate layer forms around each nanoparticle thus preventing coalescence of sulfide particles, stabilizing their size, and precluding oxidation.
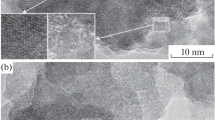
Indeed, transmission electron microscopy revealed an amorphous shell (Fig. 3) on lead sulfide nanoparticles deposited from reaction mixture 1 (Table 1). It was found experimentally earlier that such carbon-containing shell forms on the nanoparticles of silver sulfide Ag2S [23, 24] and Ag@Ag2S [25]. No protective shell was detected on the surface of nanoparticles isolated from solutions containing Trilon B [26].
The average particle size D and phase composition of powders 1–4 (Table 2) deposited with sodium citrate not changed upon long-term storage.
To elucidate the role of complexing agent, we studied nanopowders 5–9 deposited from aqueous solutions of lead acetate Pb(OAc)2 or lead nitrate Pb(NO3)2, and sodium sulfide Na2S using Trilon B after their long-term storage. Figure 4 exemplifies the typical X-ray diffraction patterns of PbS nanopowders prepared from reaction mixtures 6 and 8 containing Pb(AcO)2 or Pb(NO3)2, Na2S, and Trilon B and the same nanopowders after keeping.
Effect of exposure to air duration on the phase composition of nanopowders deposited from reaction mixtures 6 and 8 (Table 1) with Trilon B. (a): (1) nanopowder 6, D = 19 ± 2 nm; (2) exposure to air for 1050 days, 36 wt % PbS + 64 wt % PbSO4; (3) exposure to air for 2070 days, 16 wt % PbS + 84 wt % PbSO4. (b): (4) nanopowder 8, D = 15 ± 1 nm; (5) exposure to air for 1000 days, 18 wt % PbS + 82 wt % PbSO4; (6) exposure to air for 2030 days, 12 wt % PbS + 88 wt % PbSO4.
The average size of PbS nanoparticles in nanopowders 6 and 8 obtained with Trilon B (Table 1) is 19 ± 2 and 15 ± 1 nm respectively. This is 10–12 nm larger than particle size in nanopowders 1 and 3 prepared with sodium citrate.
The X-ray diffraction patterns of nanopowder 6 (Fig. 4a) after its exposure to air for 1050 and 2070 days show diffraction reflections of orthorhombic lead sulfate PbSO4 along with reflections of cubic PbS. The content of sulfate PbSO4 in nanopowder 6 after 1050- and 2070-day exposure was 64 and 84 wt %, respectively
The X-ray diffraction patterns of nanopowder 8 (Fig. 4b) deposited from solutions of lead nitrate, sodium sulfide, and Trilon B after its exposure to air for 1000 and 2030 days also contain reflections of orthorhombic sulfate PbSO4 along with the weak reflections of cubic sulfide PbS. The content of sulfate PbSO4 in nanopowder 8 after 1000 and 2030-day exposure is 82 and 88 wt %, respectively.
The comparison of phase composition changes for nanopowders 1–4 obtained with sodium citrate and nanopowders 5–9 obtained with Trilon B shows that, other conditions being equal, sodium citrate provides higher stability of phase composition of nanocrystalline lead sulfide on exposure to air than Trilon B. Let us note that lead nitrate Pb(NO3)2 is most frequently used for the preparation of nanostructured PbS with different morphology of rather large particles [27, 28]. The influence of morphology and size of nanoparticles on properties of related cubic titanium monoxide has been demonstrated in [29].
The synthesis of lead sulfide nanopowders 10–14 using Pb(OAc)2, Na2S, and Trilon B as complexing agent allows preparation of PbS nanoparticles in a wide size range (from 25 to 100 nm and more). Figure 5 represents the X-ray diffraction patterns of PbS nanopowders obtained from reaction mixtures 11, 13, and 14 and the same nanopowders after long-term storage.
Effect of duration for exposure to air on the phase composition of nanopowders deposited from reaction mixtures 11, 13, and 14 (Table 1) with Trilon B. (a): (1) nanopowder 11, D = 38 ± 3 nm; (2) exposure to air for 1010 days, 69 wt % PbS + 31 wt % PbSO4; (3) exposure to air for 2040 days, 40 wt % PbS + 60 wt % PbSO4. (b): (4) nanopowder 13, D = 70 ± 5 nm; (5) exposure to air for 1020 days, 73 wt % PbS + 27 wt % PbSO4; (6) exposure to air for 2010 days, 63 wt % PbS + 37 wt % PbSO4. (c): (7) nanopowder 14, D = 90 ± 10 nm; (8) exposure to air for 1000 days, 98 wt % PbS + 2 wt % PbSO4; (9) exposure to air for 2010 days, 90 wt % PbS + 10 wt % PbSO4.
Nanopowder 11 deposited from equimolar solutions of Pb(OAc)2 and Na2S with Trilon B contained only cubic (space group \(Fm\overline 3 m\)) lead sulfide PbS (Fig. 5a). After its storage for 1010 days, X-ray diffraction pattern displays, along with diffraction reflections of PbS, the reflections of orthorhombic sulfate phase PbSO4, whose content was ~31 wt %. After storage of this nanopowder for 2040 days, the content of lead sulfate PbSO4 increased to ~60 wt %. Addition of acetic acid in reaction mixture favors the long-term stability of phase composition of nanopowders 13 and 14 prepared from Pb(OAc)2 and Na2S with Trilon B. The addition of acetic acid that reduces solution pH seems to decrease the possibility of formation of hydroxo complexes and consequently decreases the amount of admixture oxygen and increases the stability of particle phase composition. The growth of Trilon B concentration in reaction mixtures 13 and 14 from 65 to 100 mmol/L considerably enhanced the stability of PbS powders. Powder 13 after keeping for 1020 and 2010 days contained ~27 and ~37 wt % PbSO4, respectively (Fig. 5b). Powder 14 prepared using 100 mmol/L Trilon B after keeping for 1000 and 2010 days contained less than ~2 and less than ~10 wt % lead sulfate PbSO4, respectively (Fig. 5c).
The comparison of the character of changes in X‑ray diffraction patterns for PbS nanopowders deposited from reaction mixtures Pb(OAc)2 and Na2S with Trilon B displays that smaller lead sulfide nanoparticles are less stable, i.e., undergo oxidation over shorter time. This is caused by the lack of amorphous protective shell on nanoparticles prepared with Trilon B.
Figure 6 exemplifies the change in phase composition of nanopowders 1 and 10 (Table 1) depending on keeping duration. Both nanopowders were obtained by deposition from aqueous solutions of lead acetate and sodium sulfide, but complexing agent used was sodium citrate (Na3Cit) for nanopowder 1 and Trilon B for nanopowder 10. The figure shows that nanopowder 1 prepared with sodium citrate retains its phase composition on storage in air and contains only PbS. Nanopowder 1 prepared with Trilon B upon long-term exposure to air undergoes gradual oxidation to change composition from initial PbS to PbSO4 on keeping longer than 2500 days.
Change in the phase composition of nanopowders 1 and 10 (Table 1), deposited from aqueous solutions of lead acetate and sodium sulfide with sodium citrate Na3Cit or Trilon B, respectively, on exposure to air duration.
Let us note that only lead sulfate PbSO4 was detected as oxidation product after long-term exposure of lead sulfide nanopowders to air, while PbSO3 and PbO ∙ PbSO4 phases were not observed. The formation of PbSO3 and PbO ∙ PbSO4 phases proceeds only on heating lead sulfide nanopowders to 423–523 K [4].
The average size D of nanoparticles for powders 5–14 slightly changed upon long-term storage due to oxidation (Table 2). The size of lead sulfate nanoparticles (DPbS) slightly decreased on storage, while the size of resultant lead sulfate PbSO4 nanoparticles is larger than DPbS, and slightly rises upon increase in keeping duration. As a whole, the change in size for PbS nanoparticles upon exposure to air is small (±2 nm) and indicates the stability in their size. This feature agrees well with the conclusions of work [14] on the high stability of size for lead sulfide nanoparticles.
CONCLUSIONS
Thus, the study of stability of PbS nanopowders upon long-term exposure to air has shown that the size of nanoparticles remains almost constant and stable. Effect of exposure to air on the size of nanoparticles of other sulfide nanopowders requires an independent study.
Lead sulfide nanopowders prepared using sodium citrate Na3Cit, which behaves as both complexing and stabilizing agent, have the most stable phase composition. The stabilizing role of Na3Cit is due to its ability to form a shell on the surface of nanoparticles that prevents lead sulfide oxidation. The phase composition of PbS nanoparticles prepared with sodium citrate remains unchanged for about six years.
The phase composition of PbS nanopowders prepared with Trilon B upon exposure to air changes owing to gradual oxidation of lead sulfide into lead sulfate.
Lead sulfide nanopowders prepared with sodium citrate due to stability of their size and phase composition upon long-term keeping in air are preferable for possible application in devices and equipment based on nanostructured PbS.
REFERENCES
Y. Noda, S. Ohba, S. Sato, and Y. Saito, Acta Crystallogr. B 39, 312 (1983).
W. W. Scanlon, J. Phys. Chem. Solids 8, 423 (1959).
S. I. Sadovnikov, A. I. Gusev, and A. A. Rempel, Russ. Chem. Rev. 85, 731 (2016). https://doi.org/10.1070/RCR4594
S. I. Sadovnikov, A. A. Rempel, and A. I. Gusev, Nanostructured Lead, Cadmium and Silver Sulfides: Structure, Nonstoichiometry and Properties (Springer, Heidelberg, 2018). https://doi.org/10.1007/978-3-319-56387-9
J. N. Zemmel, J. D. Jensen, and R. B. Schoolar, Phys. Rev. A 140, 330 (1965).
G. Bauer and H. Clemens, Semicond. Sci. Technol. 5, S122 (1990).
H. Preier, Semicond. Sci. Technol. 5, S12 (1990).
A. M. Malyarevich, V. G. Savitskia, M. S. Gaponenko, et al., Proc. SPIE, Intern. Conf. on Lasers, Applications, and Technologies (2005), Intern. Soc. Opt. Eng., 6054, 60540Q1 (2006). https://doi.org/10.1117/12.660806
A. Slonopas, N. Alijabban, C. Saltonstall, et al., Electrochim. Acta 151, 1409 (2014). https://doi.org/10.1016/j.electacta.2014.11.021
S. Kumar, Nano Res. Appl. 1, No. 1 (2015). https://nanotechnology.imedpub.com/archive.php.
H. Zogg, A. Fach, C. Maissen, et al., Opt. Eng. 33, 1440 (1994). https://doi.org/10.1117/12.165808
S. B. Qadri, A. Singh, and M. Yousuf, Thin Solid Films 431–432, 506 (2003). https://doi.org/10.1016/S0040-6090(03)00245-1
S. I. Sadovnikov, A. I. Gusev, and A. A. Rempel, JETP Lett. 89, 238 (2009). https://doi.org/10.1134/S0021364009050051
S. I. Sadovnikov, J. Alloys Comp. 788, 5869 (2019). https://doi.org/10.1016/j.jallcom.2019.02.244
Yu. Yu. Lur’e, Handbook on Analytical Chemistry (Khimiya, Moscow, 1967) p. 101 [in Russian].
P. Patnaik, Dean’s Analytical Chemistry Handbook (McGraw-Hill, New York, 2004), p. 1280. ISBN 978-0071410601
A. I. Gusev, Nanomaterials, Nanostructures, and Nanotechnologies (Fizmatlit, Moscow, 2009) [in Russian]. ISBN 978-5-9221-0582-8
A. I. Gusev and A. S. Kurlov, Physics of Metals and New Technologies 30, 679 (2008).
X'Pert HighScore Plus, Version 2.2e (2.2.5), PANalytical B.V. Almedo, the Netherlands.
Match! Version 1.9a. Phase Identification from Powder Diffraction, Crystal Impact.
R. Chen, N. T. Nuhfer, L. Moussa, et al., Nanotecnology 19, 455604 (2008). https://doi.org/10.1088/0957-4484/19/45/455604
S. I. Sadovnikov, Russ. J. Inorg. Chem. 64, 1309 (2019). https://doi.org/10.1134/S0036023619100115
S. I. Sadovnikov, A. I. Gusev, E. Yu. Gerasimov, and A. A. Rempel, Chem. Phys. Lett. 642, 17 (2015). https://doi.org/10.1016/j.cplett.2015.11.004
S. I. Sadovnikov and A. I. Gusev, Eur. J. Inorg. Chem. No. 31, 4944 (2016). https://doi.org/10.1002/ejic.201600881
S. I. Sadovnikov, A. I. Gusev, E. Yu. Gerasimov, et al., Inorg. Mater. 52, 441 (2016). https://doi.org/10.1134/S0020168516050149
S. I. Sadovnikov and A. I. Gusev, Russ. J. Gen. Chem. 84, 173 (2014). https://doi.org/10.1134/S1070363214020017
S. Wang, A. Pan, H. Yin, et al., Mater. Lett. 60, 1242 (2006). https://doi.org/10.1016/j.matlet.2005.10.116
Q.-L. Qing-Li Huang, Chen Hu, et al., Mater. Lett. 64, 1891 (2010). https://doi.org/10.1016/j.matlet.2010.05.048
A. A. Valeeva, K. A. Petrovykh, H. Schroettner, and A. A. Rempel, Inorg. Mater. 51, 1132 (2015). https:// doi: 10.1134/S0020168515110138
ACKNOWLEDGMENTS
The author thanks Professor A.I. Gusev for useful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declare no conflict of interest.
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Sadovnikov, S.I. Effect of Exposure to Air on the Phase Composition and Particle Size of Nanocrystalline Lead Sulfide. Russ. J. Inorg. Chem. 65, 812–819 (2020). https://doi.org/10.1134/S0036023620060170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620060170