Abstract
The equilibrium acquisition times in chromium(III)‒organic ligand systems were determined under various conditions. Chromium(III) compounds with picolinic acid Cr(C6H4NO2)3 ⋅ H2O, nicotinic acid CrOH(C6H4NO2)2 ⋅ 2H2O, and citric acid CrC6H5O7 ⋅ 3H2O were isolated; their gravimetric and thermogravimetric analyses carried out; and IR spectra measured. The major chromium(III) complex species with the above-listed acid anions in solutions were found to have a (1 : 1) stoichiometry using isomolar series; their stability constants were determined by potentiometric measurements. The electronic absorption spectra of the systems were analyzed. The photometric determination of chromium picolinate in solutions was developed, the solubility constant KS was determined (log KS = –21.52 ± 0.29), quantum-chemical calculations carried out for a gaseous molecule, and energy and geometric parameters calculated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Investigation of systems containing chromium(III) salts and biologically active organic compounds is of interest both for science and for practical needs. Chromium(III) ions play a role in biological processes, and many of chromium(III) compounds with organic acids (aliphatic and aromatic acids, hydroxy acids, and amino acids) are widely used in medicine and the food industry. Chromium is central to sugar metabolism and indispensable in the treatment of non-insulin-dependent type II diabetes. The biological role of chromium(III) picolinate consists in its involvement in the utilization of glucose by insulin and the prevention of depression. Unlike chromium(VI) complexes, most chromium(III) complexes are not cytotoxic and not mutagenic, likely, due to their low cellular permeability associated with their octahedral geometry and kinetic inertness.
It is topical to study chromium(III)‒organic acid systems, where the course of complex formation of the Cr3+ ion is complicated by hydrolysis, polymerization, and substitution of ligands in the inner sphere.
The main property of chromium(III) complexes is their kinetic stability to substitution reactions in aqueous solutions; it is just for this reason that so many chromium(III) complexes have been isolated. Many reactions involving chromium(III) ions in aqueous solutions occur slowly at room temperature. Chromium(III) complexes are proven to be almost inert, and the ligand-exchange scheme is recognized. Kornev and Mikryukova [1] show that the experimentally determined ligand-substitution rate in the inner sphere of kinetically inert aqua complex [Сr(Н2O)6]3+ (the half-life of water molecules for it is 1‒3 days [2]) is the rate at which the outer-sphere complex [Cr(H2O)6]Hn – iL (charges omitted), which is rapidly formed as an ion pair (Hn–iL is any ligand species), converts to an inner-sphere complex:
The solutions are thereby acidified; a proton can be detached either from the ligand or from the water molecule of the chromium(III) aqua complex.
Syntheses of chromium(III) salts of organic ligands are documented. Gabriel et al. [3] describe the preparation of Na3[Cr(C6H5O7)2] ⋅ 8.5H2O, a salt containing a complex anion, from chromium(III) nitrate, citric acid monohydrate (H3Cit ⋅ H2O) in the molar ratio 1 : 3, and aqueous NaOH at рН 5.5 [3]. This compound is reported to be violet. Boynton and Evans in the patent [4] describe a method for preparing chromium(III) picolinate from an aqueous solution of CrCl3 ⋅ 6H2O and picolinic acid C6H5NO2 (HPic): a mixture of reagents is boiled for 15 min and cooled, which is followed by crystallization, filtration, and recrystallization to obtain dark pink crystals. Yellow rhodium(III) and iridium(III) picolinate crystals of having a similar composition MPic3 ⋅ H2O were prepared from solutions of metal chlorides and picolinic acid under heating for 4 h [5]. The crystal structures of both complexes were determined by X-ray diffraction. Picolinate ligands in complexes are coordinated to the center as bidentate N,O-donors to form five-membered rings. The water molecule is bonded to the carboxy moieties of two neighboring MPic3 molecules and performs as a bridge between individual complex molecules. Chakov et al. [6] describe the preparation of dimeric picolinate Cr2(μ-OH)2(Pic)4 ⋅ 5H2O and its NMR spectrum in dimethyl sulfoxide.
An overview of the literature on chromium(III) nicotinate preparation points to a great dependence of the salt composition on synthetic conditions. The trinuclear chromium(III) complex Na[Cr3O(HNic)6(H2O)3](ClO4)8 ⋅ HNic ⋅ 6H2O was prepared by reacting nicotinic acid (HNic), chromium perchlorate hexahydrate, and sodium perchlorate in aqueous solution; its composition was derived from analytical and single-crystal X‑ray diffraction data [7]. Nicotinic acid is bonded to chromium only through the carboxy oxygen atom. Green et al. [8] synthesized trans-[Cr(1,3-pn)2(Nic)2]Cl ⋅ 4H2O (1,3-pn = 1,3-propanediamine) orange crystals. Deuterium NMR spectroscopy showed that two trans-Nic−-anions are coordinated through carboxy groups. Vicens et al. [9] describe Cr2Nic3(OH)3 ⋅ 4H2O and Cr2(HNic)3Cl6 ⋅ 6H2O nicotinates and new chromium(III)‒nicotinic acid‒amino acid ternary complexes with histidine Cr(L-His)(HNic)Cl3 ⋅ 5H2O and the cysteine anion Cr(L-Cis−)(HNic)Cl2 ⋅ 4H2O (the final рH is 3.0). Their characteristics were found by physical measurements; in all cases nicotinic acid is bonded to the chromium(III) atom through the carboxy group.
Cooper et al. [10] prepared CrNic2 ⋅ 4H2O, a yellow crystalline compound, and CrNic2OH ⋅ 3H2O, a blue solid compound. It was reported that nicotinic acid in a chromium(II) complexes is bonded only via the pyridine nitrogen, in chromium(III) complexes, via the carboxylate. Compounds with Cr−N bonds are yellow or red; compounds with Cr−O bonds are green or blue. Chromium(III) strongly binds OH groups and Н2О molecules in aqueous solutions to form polymeric complexes; on the whole, chromium(III) complexes with nicotinate ion can be olates.
Broadhurst et al. [11] studied the structures of CrPic3 and three samples with nicotinate ion (CrPic2Nic, CrPicNic2, and CrNic3) using liquid-phase NMR and solid-phase Fourier method. None of the samples with nicotinate ions were crystalline; each product had its own color. The olate structure correlates with the observation that CrPicNic2 and CrNic3 are relatively low soluble in H2O and DMSO, and it can help to explain the observed blue and green colors. The absolute energies of various molecular conformations were calculated for prepared compounds (the calculations referred to room temperature and the gas phase). A comparison of the relative stabilities of the four conformations indicates a greater stability of the molecular conformation CrPic3. There is not much data on chromium(III) complex formation with organic ligands in solution in the literature; there is also information about mixed complex formation. The stability constant of a chromium(III) picolinic acid complex of composition [CrPic2]+ (logβ2 = 10.22, I = 0.5) can be found in [12]. Kornev and Mikryukova [13] studied heteroligand complex formation of chromium(III) in Cr3+‒HnL‒H2Sal systems, where HnL stands for monoamine carboxymethyl complexones (methyliminodiacetic acid, β-hydroxyethyliminodiacetic acid, or nitrilotriacetic acid) and H2Sal stands for salicylic acid; stability constants were determined for 1 : 1 : 1 complexes. The composition and stability constants were determined for mono- and biligand chromium(III) complexes with carboxymethyleneamine complexones and with aliphatic (citric and tartaric acids) and aromatic hydroxycarboxylic acids using spectrophotometric data and mathematical simulation [1].
Studies into physicochemical and biological properties of chromium(III) picolinate are now underway. The use of chromium(III) picolinate as a dietary supplement is causing more and more discussion. Seven new derivatives Cr(R–Pic)3 (R = H, Br, CF3, Cl, COOH, CH3, 5-OH, and 3-OH) were prepared and characterized to be compared with chromium(III) picolinate [14]. These chromium(III) complexes have no considerable effects on the blood glucose, serum insulin, total cholesterol, etc. in diabetic mice. The above-listed substituents are incapable of distinctly improve the biological activity of CrPic3, so the rationale for use of chromium(III) picolinate as a dietary supplement is doubtful.
Uddin et al. [15] and Rodriguez et al. [16] studied the electrochemical reduction of CrPic3 in the presence of proton donors, such as ascorbic acid, benzoic acid, and acetic acid; the exchange of inner-sphere water molecules of the complex ion [CrPic(H2O)4]2+ with outer-sphere H2O molecules is studied.
EXPERIMENTAL
The spectra of solutions of the studied systems were recorded on a Leki SS2107UV spectrophotometer; the optical density was measured on a KFK-2-UKhL 4.2 photocolorimeter with the absorbing layer thickness l = 10 mm; pH in solutions was determined on a 673 ion meter equipped with a glass electrode, which was calibrated against hydrochloric acid solutions whose pH was known and ionic strength was determined. The thermoanalytical curves of prepared salts were recorded on a Netzsch STA 449 F1 thermal analyzer under the following conditions: an Al2O3 crucible, 10 K/min heating rate, and 80 mL/min air flow. IR spectra were recorded on an Agilent Cary 630 FTIR spectrometer in the frequency range 400–4000 cm–1.
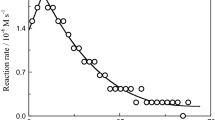
In view of the high inertness of chromium(III), one who intends to prepare its compounds with organic ligands or to study complex formation in solution, needs to know the time required by the system to attain equilibrium. The effect of time on the chromium(III) chelate formation rate was studied as the evolution of pH in solutions over time in the Cr(NO3)3‒H3Cit (cCr = cCit = 0.0125 mol/L; pH0 3.5; Vtot = 15 mL) system taken as an example (Fig. 1).
Figure 1 implies that the Cr(NO3)3–H3Cit system at room temperature attains equilibrium in 2–3 days. When a solution is heated on a water bath (Vtot ≈ const), this system attains equilibrium in 3–3.5 h (pH changes from 3.5 to 2.5 due to Cr3+ hydrolysis increasing under heating). Based on the electronic absorption spectra of this system recorded after solutions were boiled for various times (the solution volume was maintained constant), we concluded that complex formation and hydrolysis equilibria (Cf. the electronic absorption spectra of the Cr(NO3)3–H2O system under the same conditions) are attained in 20 min (pH changes from 3.5 to 2.1). In all cases, the solutions were noticed to change color from blue–violet belonging to hexaaqua ions [Cr(H2O)6]3+ to dark blue; the occurrence of complex formation with an organic ligand was thereby proved. We proceeded from the related literature and our determined conditions for equilibrium acquisition in systems containing chromium(III) and an organic ligand to choose the method for preparing salts and the conditions for studying chromium(III) complex formation with citric acid, picolinic acid, and nicotinic acid.
Chromium(III) citrate was prepared by reacting chromium(III) nitrate nonahydrate with citric acid (the molar component ratio was 1 : 1):
For this purpose, to 5 mL of a solution containing 3.32 g of Cr(NO3)3 ⋅ 9H2O, added was 5 mL of a citric acid solution (1.74 g of Н3Cit ⋅ H2O). In the thus-prepared blue–violet clear solution (pHmix 1.36), pH was adjusted to ~3.7‒4.0 by concentrated aqueous NaOH. The mixture was heated on a water bath for 3 h, cooled, and allowed to stand for 24 h (рН 2.3); chromium(III) citrate was salted out by acetone. The produced mass was washed with acetone and then it was dried in air.
Chromium(III) picolinate was prepared by reacting chromium(III) nitrate nonahydrate with picolinic acid in the molar ratio 1 : 3 (Vmix ~ 30 mL):
In the thus-prepared clear solution, pH was adjusted to 3.40 by 2.5 М NaOH. While the solution was allowed to stand (for 2 days), a dark pink precipitate settled; the precipitate was washed with water and dried in air. Chromium picolinate was also prepared by heating a mixture of the initial components on a bath (for 2 h) at pH 1.9‒3.5. The salts prepared in this pH range had identical compositions.
To prepare chromium(III) nicotinate (the initial component ratio 1 : 3), to 15 mL of a solution containing 2.86 g of Cr(NO3)3 ⋅ 9H2O, added were 15 mL of a slightly warmed solution of 2.77 g of nicotinic acid and 4.30 mL of 2.5 М NaOH. The thus-prepared grey–green clear solution was kept on a water bath for 3 h, cooled, and allowed to stand for 24 h. The solution with the precipitate acquired grey color; it was once more kept on a water bath; after the solution was cooled (рН 3.20), the settled precipitate was filtered off, washed with cold water, and air dried.
The synthesized chromium(III) salts were analyzed gravimetrically for water and chromium oxide. For this purpose, salts were kept at 130°С (for 2 h) to remove water and then at 900°С for 2–3 h for Cr2O3 to form. The water, organic ligand, and chromium oxide in the salts were also determined thermogravimetrically. Satisfactory convergence of crystal water determinations in chromium(III) citrate and chromium(III) picolinate by thermogravimetry (Tmax = 110 and 118°С, respectively) and gravimetry (130°С) allowed their averaging. A good convergence between gravimetric and thermogravimetric data was observed for chromium(III) oxide determinations in all salts. The averaged results of gravimetric and thermogravimetric analyses of the salts appear in Table 1. A good convergence is observed between the calculated and experimentally determined contents of water, ligand, and chromium oxide in the salts. The thermolysis of chromium(III) citrate CrCit ∙ 3H2O in air involves several stages as shown by the weight change on thermoanalytical curves: endothermic dehydration occurs in the range 70−240°С (Tmax = 110°C); the exotherm at 240−440°С (Tmax = 410°C) corresponds to the complete thermal destruction of citrate ion followed by Cr2O3 formation:
For chromium(III) hydroxonicotinate CrOHNic2 ∙ 2H2O, the set of endotherms at 70−340°С (Tmax = 225°C) on the thermoanalytical curve corresponds to the dehydration and partial sublimation of nicotinic acid. The exotherm in the range 340−520°С (Tmax = 446°C) is due to the complete burnout of nicotinate ion in air and the formation of chromium(III) oxide. The water loss by chromium(III) picolinate CrPic3 ∙ H2O occurs with an endotherm in the range 70−210°С (Tmax = 118°C). In the range 320−570°С (Tmax = 370, 518°C), the destruction of the anion and the onset of chromium(III) oxide formation are observed.
The solubility constant was determined for the synthesized chromium(III) picolinate chromium CrPic3 ∙ H2O: KS = [Cr3+] ∙ [Pic−]3. Since the salt is poorly soluble, initial solutions with sufficiently high hydrochloric acid concentrations (0.3−1 mol/L), pH 0.52‒0, and ionic strength I = 1 were taken to determine its solubility. From Fig. 2, one can see the yield of the CrPic2+ complex in solutions in the specified pH range is as low as ~(8–2)%. So, ignoring complex formation in acid solutions, the equilibria and the relevant constants taken into account for a saturated chromium picolinate solution were as follows:
Cr3+ (α0) and СrPic2+ (α1) species yields as a function of pH (cL = 0.01mol/L, logB1 = 5.15, logβ1 = 5.58; the “Yield of complex” program [17]).
From equilibrium (1), one can see that in a saturated solution, cCr = csalt (csalt is the solubility of the salt, mol/L); then, the mass balance equation for the metal ion is
From expression (3), for Kh1 we find [CrOH]2+ = Kh1 [Cr3+]/h (h is equilibrium hydrogen ion concentration [H+]); the hydrolysis function ω = 1 + Kh1/h, and Kh1 is the Cr3+ hydrolysis constant taken equal to 7.1 × 10−5 [1]. From Eq. (4) we obtain [Cr3+] = csalt/ω. From Eq. (5), which is the mass balance equation for the ligand with account to equilibrium (1),
where f = 1 + В1h (В1 is the protonation constant of the picolinic acid anion), we find: [Pic−] = cPic/f = 3csalt/f. In function f calculations, the hydrogen ion concentration h was set equal to the initial concentration of HCl solutions (I = 1), since the CrPic3 ∙ H2O solubility (about 10‒3 mol/L) is far lower than the initial HCl concentration (0.3‒1 mol/L). Substituting [Cr3+] = csalt/ω and [Pic−] = 3Csalt/f to the expression KS = [Cr3+] ∙ [Pic−]3, we have
The salt content in saturated chromium(III) picolinate solutions (\(C_{{{\text{HCl}}}}^{0}\) = 0.3‒1 mol/L) was determined photometrically at λeff = 440 or 540 nm; calibration solutions (cHCl = 0.5 mol/L, pH 0.30; I = 1) were prepared using a standard CrPic3 ∙ H2O solution with the concentration 2.36 × 10−3 mol/L. the calibration curves for CrPic3 solutions at 440 (Fig. 3) and 540 nm have good correlation coefficients (0.996 and 0.995, respectively). To determine the solubility of the salt, to an aliquot of its saturated solution, appropriate volumes of 1 М HCl solution and 2 М NaCl solution (Vtot = 6 mL) were added to provide cHCl = 0.5 mol/L and I = 1 as in the calibration solutions. According to the yields of Cr3+ and CrPic2+ species (Fig. 2), 0.5 М HCl solutions (pH 0.30) contain ~95% aquated chromium(III) ions, which are responsible for absorption in the solutions.
Table 2 data compiles data on the determination of chromium(III) picolinate solubilities in solutions with \(C_{{{\text{HCl}}}}^{0}\) = 0.3‒1 mol/L and the calculated solubility constants for CrPic3 ⋅ H2O (I = 1).
Complex formation in Cr3+‒HnL (HnL = HPic, HNic, and H3Cit) systems was studied using isomolar series and potentiometrically at the ionic strength I = 0.3 adjusted by NaNO3 solution (the background electrolyte has a common ion with the solution Cr(NO3)3). The isomolar series photometric method showed that the 1 : 1 complex is the major species in all systems. Figure 4 illustrates an isomolar series for the Cr3+‒HNic system as an example.
In view of the fact that photometric data point to the dominance of 1 : 1 complex species in the systems studied, the metal-to-ligand ratio was set equal to 1 : 1 in potentiometric determination of stability. To a mixture of a Cr(NO3)3 solution with the free acidity cH = 1 × 10−3 mol/L (HNO3) and a solution of the relevant organic acid, added were various NaOH volumes, and the mixed solutions were exposed for 3 days for equilibration. Table 3 compiles data on the preparation of solutions, pH measurements in them, and calculations of logarithmic stability constants in the Bjerrum 1 program [17] for [CrPic]2+ (logβ1 = 5.58 ± 0.26). For [CrNic]2+ and [CrCit] complex ions, potentiometric logβ1 is 4.14 ± 0.26 and 7.15 ± 0.11, respectively.
For Сr(NO3)3−H2O, Сr(NO3)3−HPic and Сr(NO3)3−HNic systems (cCr = cL = 0.025 mol/L; Vtot = 10 mL; I = 0.3 (NaNO3)), electronic absorption spectra were recorded in the visible. In all solutions, рН was adjusted by aqueous HNO3 or NaOH; the solutions were allowed to stand for 3 days for equilibration (рН 2.9‒3.1). Figure 5 shows the absorption spectra of chromium(III) nitrate and a mixture of a metal salt and picolinic acid. The absorption spectra of Сr(NO3)3−HNic and Сr(NO3)3−H3Cit systems resemble the spectrum of the Сr(NO3)3−HPic system.
The quantum-chemical calculations of chromium(III) picolinate were performed in the Gaussian 09 software [18] in terms of density functional theory (DFT) with the functional B3LYP and the basis set 6‑31G (d). Full optimization of the molecule was used to find stationary points on the potential energy surface. As a result, calculated were a suggested structure of chromium(III) picolinate in the gas phase (the positions of the central atom and ligands in the space, bond lengths, and bond angles), the full and relative energies of a gaseous molecule, and the IR spectrum of chromium(III) picolinate in order to compare it with the experimentally measured spectrum. The calculated structure of the molecule under study is shown in Fig. 6.
The results of quantum-chemical calculations of the full energy and relative energy (the difference between the full molecular energy Еfull and the zero-point energy of vibrations ЕZPE) of chromium(III) picolinate appear in Table 4.
The geometric parameters of the structure (bond lengths and bond angles in planar five-membered rings in a chromium(III) complex with picolinic acid) were calculated in agreement with Fig. 6 (Tables S1, S2). A comparison of the calculated and experimentally measured IR vibration frequencies of a chromium(III) picolinate molecule is shown in Table S3.
RESULTS AND DISCUSSION
When a ligand is inserted into chromium(III) salt solutions, the following is observed: acidity changes; the absorption peaks of chromium(III) complexes experience a small shift relative to those of hydrated metal ion; and molar absorption coefficients differ from the molar absorption coefficients of the precursors. The absorption peaks, the respective optical densities, and the differences between these quantities in Сr(NO3)3−H2O and Сr(NO3)3−HNic(HPic)−H2O systems with the same component concentrations and similar pH values appear in Table 5 (hypsochromic shifts for ∆λ are denoted by minus signs). Low molar absorption coefficients of solutions containing aquated chromium(III) ions and their complexes at wavelengths 411–399 and 575–544 nm (17.24–26.76 and 14.08–20.40, respectively) signify that the bands at these wavelengths belong to d–d electron transitions in Cr3+ (d3). The spectra of the studied systems feature two absorption bands that can be assigned to d–d electron transitions in Cr3+ (d3): 4A2g → 4T1g (in the range 399−411 nm) and 4A2g → 4T2g (in the range 544−575 nm); this assignment is supported by [9, 19]. From Table 5 one can infer that the absorption peaks (λmax) of aquated chromium(III) ions in the systems with ligands experience a hypsochromic shift, indicating an increased splitting energy of chromium(III) atomic d orbitals by the ligand field compared to the split in an aquated chromium(III) ion. This split is greater in the case of the picolinate ion, which forms a more stable chelate complex with Cr3+ than the nicotinate ions does.
Table 6 lists stability constants for chromium(III) complexes with citric, picolinic, and nicotinic anions as determined in this work; for comparison, stability constants borrowed from the literature are given for iron(III) complexes with the same ligands. The closeness of the ionic radii of tervalent chromium and iron is responsible for the closeness of stability constants of their complexes. A lightly higher stability of Fe3+ (d5) complexes compared to Cr3+ (d3) complexes with oxygen- and nitrogen-containing ligands can be explained by a higher electrostaticity of the iron(III) ion and its greater affinity to oxygen and nitrogen donor atoms. The order of increasing stabilities of chromium(III) complexes ([CrNic]2+, [CrPic]2+, and [CrCit]) agrees with the anion charges and acid basicities (logB1 = 4.81, 5.15, and 5.49, respectively).
From the quantum-chemical calculations for a gaseous chromium(III) picolinate, one can infer that the CrPic3 species resides in the space in the form of an octahedron, just as expected. The chromium(III) central ion forms three planar five-membered rings via the oxygen and nitrogen donor atoms of bidentate picolinic acid ligands. The greater negative value obtained for the energy of a chromium(III) picolinate molecule (Table 4) signifies a high stability of this compound. Presumably, this might arise from the strong bond formed by the chromium(III) central atom with the oxygen and nitrogen donor atoms of pyridine-2-carboxylic acid in five-membered rings.
The bond angles sum in a planar pentagon is 540.0°, and the sum of calculated bond angles in five-membered rings 1, 2, and 3 in a chromium(III) picolinate molecule is 543.4°, 539.9° and 539.7° (Table S2), respectively; this indicates their flat arrangement in space, taking into account the error. The small values of bond angles O2Cr1N1, O4Cr1N2, N3Cr1O6 (78°–83°), differing from 108° (the bond angle in a planar triangle), can be explained as follows: given an octahedral coordination, Cr‒O and Cr‒N bonds are elongated, and the bond angle thereby decreases when the chromium(III) ion forms five-membered rings. These bond lengths in Table S1 are in bold. Hakimi [21] used X-ray crystallography to find structural characteristics of CrPic3 ∙ H2O (bond lengths and bond angles) that are close to our quantum-chemical calculations (Table 7). The discrepancy between the bond lengths in a chromium(III) picolinate molecule obtained by theoretical calculations in Gaussian 09 and the X-ray crystallography data [21], is 0.01‒0.06 Å for Cri‒Nn bonds and 0.04‒0.07 Å for Cri‒On bonds; this may be regarded to be satisfactory. The authors mention that a chromium atom in a picolinate crystal coordinates three nitrogen atoms and three oxygen atoms, and it has a distorted octahedral geometry. Each water molecule performs as a hydrogen-bond bridge to connect two neighboring complexes. Semanti et al. [5] determined the structure of rhodium(III) and iridium(III) picolinates by X-ray crystallography; the structural characteristics are close to the ones determined in this study. For example, the angle N2Cr1O4 (Fig. 6, Table S2) is 82.8° against 81.75° in rhodium(III) picolinate, 80.72° in iridium(III) picolinate, and 80.5° (in chromium(III) picolinate as reported in [5] and [21].
A satisfactory coincidence of the IR spectrum calculated in Gaussian 09 and the one measured experimentally (Table S3) indicates that the central chromium atom in chromium(III) picolinate CrPic3 ∙ H2O has an octahedral surrounding and the crystal water molecule is outside this surrounding.
REFERENCES
V. I. Kornev and G. A. Mikryukova, Vestn. Udmurtsk. Univ., Khim., No. 8, 163 (2006).
A. K. Lavrukhina and L. V. Yukina, The Analytical Chemistry of Chromium (Nauka, Moscow, 1979) [in Russian].
C. Gabriel, C. P. Raptopoulou, C. Drouza, et al., Polyhedron 28, 3209 (2009). https://doi.org/10.1016/j.poly.2009.05.077
H. Boynton, G. W. Evans, et al., US Pat. 5087623 (1992).
B. Semanti, S.-M. Peng, G.-H. Lee, and S. Bhattacharya, Polyhedron 24, 157 (2005). https://doi.org/10.1016/j.poly.2004.10.015
N. E. Chakov, R. A. Collins, and J. B. Vincent, Polyhedron 18, 2891 (1999). https://doi.org/10.1016/S0277-5387(99)00208-9
E. Gonazler-Vergara, J. Hegenauer, P. Saltman, et al., Inorg. Chim. Acta 66, 115 (1982). https://doi.org/10.1016/S0020-1693(00)85799-0
C. A. Green, R. J. Bianchini, and J. I. Legg, Inorg. Chem. 23, 2713 (1984). https://doi.org/10.1021/ic00185a032
M. Vicens, J. J. Fiol, and A. Terrbn, Inorg. Chim. Acta 192, 139 (1992). https://doi.org/10.1016/S0020-1693(00)83183-7
J. A. Cooper, B. F. Anderson, P. D. Buckley, and L. F. Blackwell, Inorg. Chim. Acta 91, 1 (1984). https://doi.org/10.1016/S0020-1693(00)84211-5
C. L. Broadhurst, W. F. Schmidt, J. B. Reeves III, et al., J. Inorg. Biochem. 66, 119 (1997).
L. G. Sillen and A. E. Martell, Stability Constants of Metal–Ion Complexes (Chemical Soc., London, 1964), Pt. 3 (1), p. 435. https://lib.ugent.be/catalog/rug01:000022724.
V. I. Kornev and G. A. Mikryukova, Khim. Fiz. Mezoskop. 7, 71 (2005).
J. Chai, Y. Liu, J. Dong, et al. Inorg. Chim. Acta 466, 151 (2017). https://doi.org/10.1016/j.ica.2017.05.041
K. M. Uddin, A. I. Alrawashdeh, T. Debnath, et al., J. Mol. Struct. 1189, 28 (2019). https://doi.org/10.1016/j.molstruc.2019.04.015
C. M. I. Rodríguez, V. Piscitelli, C. Borras, et al., J. Mol. Liq. 211, 401 (2015). https://doi.org/10.1016/j.molliq.2015.07.019
N. A. Skorik and E. B. Chernov, Computer Calculations in the Chemistry of Complexes (TGU Press, Tomsk, 2009) [in Rusian].
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 09, Revision A.01 (Gaussian, Wallingford CT, 2009).
K. V. Mezentsev and Yu. A. Mikhailenko, Vestn. Kuz. Gos. Tech. Univ., No. 6, 121 (2010).
V. V. Pal’chevskii, V. V. Khorunzhii, and V. I. Shcherbakova, Koord. Khim. 10, 1076 (1984).
M. Hakimi, J. Korean Chem. Soc. 57, 721 (2013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by O. Fedorova
Supplementary material
Rights and permissions
About this article
Cite this article
Skorik, N.A., Alimova, R.R. Chromium(III) Compounds with Some Organic Ligands. Russ. J. Inorg. Chem. 65, 13–21 (2020). https://doi.org/10.1134/S0036023620010167
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010167