Abstract—
Extraction of micro amounts of rare earth elements(III) (REE) from nitric acid solutions with diethyl 2-[(diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate (I) in dichloroethane and ionic liquid, 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide has been studied. The stoichiometry of extracted complexes has been determined, the effect of HNO3 concentration in aqueous phase on the efficiency of metal ion recovery into organic phase has been considered. It has been found that compound I in dichloroethane extracts REE(III) less efficiently than its analogs with phenyl and butyl substituents at the phosphorus atom. It has been shown that the efficiency of REE(III) extraction with compound I from nitric acid solutions increases considerably in the presence of ionic liquid in organic phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Extraction methods are widely used for the preconcentration and separation of rare earth elements (REE) [1]. The study of extraction properties of new polyfunctional organophosphorus compounds is of interest due to the promise of their use in radiochemical and analytical practice. Polydentate neutral organophosphorus compounds show high extraction ability toward REE(III) [2, 3], the most studied compounds among them are methylenediphosphine dioxides [4], (dialkylcarbamoylmethyl)diarylphosphine oxides (CMPO) [5] and their derivatives [6], N‑diphenylphosphoryl-N'-alkylureas [7], phosphorylated calixarenes [8–12], phosphorylated pyridine N-oxides [13, 14], as well as acyclic analogs of crown ethers: podands with amide [15–20] or phosphoryl [21–23] terminal groups.
The extraction ability and selectivity of phosphoryl-containing podands (PP) is considerably affected by the length of polyether chain, the structure of bridge between the ethereal oxygen atom and the PO group, and the nature of substituent at the phosphorus atom [21–23]. In certain cases, the replacement of alkylene bridge in the molecules of tetraphenylalkylenediphosphine dioxides by ethylene glycol fragments leads to increase in the extraction ability of the corresponding PP toward REE(III) [23]. Among these compounds, tetraaryl-substituted (o-phenyleneoxymethylene)diphosphine dioxides show the highest extraction ability toward actinide and REE(III) ions [23], which is superior to that for CMPO. The replacement of aryl substituents at the phosphorus atom in these compounds by alkyl ones leads to the sharp drop of extraction ability of PP toward actinides and REE in nitric acid solutions due to “anomalous aryl strengthening” (AAS) effect for extracted complexes [24]. Extraction properties of PP with alkoxy groups at the phosphorus atom were studied in much less extent [25].
In recent time, there is growing interest in the use of ionic liquids (ILs) in the processes of extraction preconcentration and separation of organic and inorganic compounds [26–29]. REE(III) extraction with bidentate neutral organophosphorus compounds [30–32] and substituted diglycolamides [33–35] considerably increases in the presence of ILs, 1-alkyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imides. In these systems, the quantitative recovery of REE(III) requires no considerable excess of \({\text{NO}}_{3}^{ - }\) ions in aqueous phase, which is necessary condition for extraction with these extractants in common organic solvents. There are no literature data on the effect of IL on metal ion extraction with PP.
The aim of this work is to study effect of IL on the efficiency of REE(III) extraction from nitric acid solutions with solutions of compound I. Therefore, we considered certain features of REE(III) distribution between aqueous HNO3 solutions and solutions of compound I in 1,2-dichloroethane and IL, 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide. We compared the data on REE(III) extraction with compound I and its structural analogs with phenyl (II) and butyl (III) substituents at the phosphoryl group.

EXPERIMENTAL
Diethyl 2-[(diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate (I) was obtained by alkylation of diethyl (2-hydroxy-5-ethylphenyl)phosphonate with (diphenylphosphoryl)methyl benzenesulfonate in the presence of sodium hydride in boiling dioxane by the previously described procedure [36]. Yield of compound I was 69%, mp = 89–91°С (benzene–hexane).
For C25H30O5P2 anal. calcd. (%): C, 63.56 H, 6.40; P, 16.93.
Found (%): C, 63.49, 63.15; H, 6.05, 6.32; P, 16.41, 16.67.
1H NMR (CDCl3, δ, ppm): 2.18 m (9H, 2CH3CH2O + Ar–CH2CH3), 3.65 q (2H, Ar–CH2CH3), 5.01 m (4H, 2CH3CH2O), 5.82 d (2H, 2JH–P = 16 Hz, OCH2P(O)Ph2), 7.96 t (1H, 3JH–H = 7.3 Hz, Ar–H), 8.30 t (1H, 3JH–H = 4.6 Hz, Ar–H), 8.52 m (6H, Ar–H), 8.76 d (1H, 2JH–P = 32 Hz, P(O)Ar–H), 9.04 m (4H, Ar–H). 31P NMR (CDCl3, δ, ppm): 18.55, 27.96.
Organic diluents used were 1,2-dichloroetane of reagent grade without additional purification and 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide (C4mimTf2N), prepared and purified by the known procedure [37]. Extractant solutions were prepared from precisely weighed sample.
Initial REE(III) aqueous solutions were prepared by dissolution of the corresponding nitrates in water followed by addition of HNO3. Initial metal ions concentration was 2 × 10–6 mol/L. Phases were contacted at ambient temperature on a rotary stirring apparatus with stirring rate 60 rpm for 1 h. It was preliminary established that this time is sufficient to reach constant values of distribution ratios (DLn) in extraction systems.
REE(III) concentration in initial and equilibrium aqueous solutions was determined by inductively coupled plasma mass spectrometry (ICP-MS) using a Thermo Elemental X-7 mass spectrometer (USA). Element content in organic phase was determined after back extraction with 0.1 M solution of hydroxyethylidenediphosphonic acid. Distribution ratio of elements was calculated as the ratio of their concentrations in organic and aqueous phases. Determination error for distribution ratio was not larger than 5%.
The concentration of Tf2N– ions in equilibrium aqueous phases was determined by inductively coupled plasma-atomic emission spectroscopy (ICP-AES) using a Thermo Jarrel Ash ICAP-61 spectrometer (USA).
The concentration of HNO3 in equilibrium aqueous phase was determined by potentiometric titration with NaOH solution, that in organic phase was determined by the same method after back extraction of HNO3 with water. The content of HNO3 in organic phase was determined by acid extraction with pure dichloroethane (blank test). The result of blank test was taken into account in computation of total concentration of HNO3 complexes with extractant in organic phase.
RESULTS AND DISCUSSION
The process of metal ion extraction from nitric acid solutions with neutral organophosphorus compounds is accompanied by HNO3 reaction with extractant. Therefore, we preliminary studied HNO3 extraction with solution of compound I. The data on HNO3 distribution between its aqueous solutions and compound I solution in dichloroethane (Fig. 1) indicate that at [HNO3] > 2.5 mol/L, the ratio of HNO3 concentration bound in complexes with the extractant to initial extractant concentration in organic phase is larger 1. Assuming that extractant (L) complexes with one or two nitric acid molecules pass into organic phase, the process of HNO3 extraction can be described by the equation:
where symbols (o) indicate the components of organic phase, i = 1 and 2 are the number of HNO3 molecules in the extracted solvate. Efficient extraction constants for HNO3 (K1 and K2) are expressed as
where a is HNO3 activity in equilibrium aqueous phase (a = [H+][\({\text{NO}}_{3}^{ - }\)]\(\gamma _{ \pm }^{2}\)), [L] is equilibrium concentration of free extractant in organic phase. The values of K1 and K2 calculated by non-linear least squares using equation:
where y is the total concentration of HNO3 complexes with extractant in organic phase, [L]ini is the initial extractant concentration, are shown in Table 1. For comparison, the table also displays K1 and K2 values for compounds II and III. Increase in the extraction ability toward HNO3 in the series of compounds I < II < III is due to increase in the donor ability of the P=O group as the electronegativity of the substituents at the phosphorus atom decreases [38].
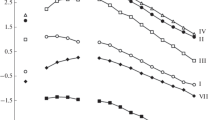
We considered the effect of HNO3 concentration in equilibrium aqueous phase on the alteration of distribution ratios of REE(III) upon extraction with solution of compound I in dichloroethane (Fig. 2). Extraction of Ho(III)–Lu(III) increases with [HNO3], while the extraction of La(III)–Tb(III) shows log DLn–[HNO3] dependences with maximum, which is owing to the salting out effect of \({\text{NO}}_{3}^{ - }\) ions and extractant binding with nitric acid, as well as change in the activity coefficients of REE(III) nitrates depending on [HNO3]. The position of maximum is shifted to the region of higher acidity of aqueous phase as atomic number (Z) of REE increases, which is caused by the growth of hydration energy of Ln3+ ions because of decrease of their ionic radii when Z rises. This leads to increase in Lu/La separation factor (βLu/La = DLu/DLa) with HNO3 concentration (Fig. 2). The similar character of DLn–[HNO3] dependence was noted for the extraction of REE(III) with solutions of compounds II and III as coordination-solvated nitrates [23].
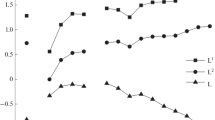
Metal : extractant stoichiometric ratio in the extracted complexes was determined by equilibrium shift method. Obtained data (Fig. 3) showed that REE(III) are extracted with this compound as a mixture of mono- and disolvates. REE(III) are extracted with solutions of compounds II and III in dichloroethane as complexes of the same stoichiometry [21]. The obtained data indicate that the nature of substituent at the phosphorus atom in compounds I–III causes no considerable change in the stoichiometry of extracted REE(III) complexes. On the basis of obtained data, the process of REE(III) extraction from HNO3 solutions of moderate concentration with compound I can be described by the equations:


Table 2 shows the efficient constants of REE(III) extraction (\(K_{1}^{{{\text{Ln}}}}\) and \(K_{2}^{{{\text{Ln}}}}\)) calculated by nonlinear least squares using equation
where γ± is activity coefficient of the corresponding REE(III) nitrate [39], f is a correction for extractant binding with nitric acid (f = 1 + K1a + K2a2). The table also shows \(K_{2}^{{{\text{Ln}}}}\) values for compounds II and III. The presented data display that the extraction ability of PP toward REE(III) increases in the series of compounds I < III < II. The high extraction ability of compound II with phenyl substituents at the phosphorus atom toward REE(III) is due to appearance of AAS effect in the systems with PP.
REE(III) extraction with compounds I–III from solutions with moderate HNO3 concentration shows a trend to decrease REE(III) extraction efficiency when Z increases. This is caused by enhancement in the stability of REE(III) complexes with hard (according to Pearson) ligands when charge density of Ln3+ ions rises owing to decrease of their ionic radii as Z increases [40].
The efficiency of REE(III) extraction with solutions of compound I from nitric acid solutions considerably increases in the presence of IL. This may be due to the growth of hydrophobicity of extracted complexes on account of displacement of \({\text{NO}}_{3}^{ - }\) ions by more hydrophobic Tf2N– ions [41]. The character of log DLn–[HNO3] dependence changes in the presence of IL in organic phase (Fig. 4). The decrease of DLn when [HNO3] rises up to 3 mol/L is caused by the drop of free extractant concentration in organic phase due to reaction of compound I with both HNO3 and HTf2N present in the system owing to marked transfer of C4mim+ and Tf2N– ions in aqueous phase [42]. Extraction of HTf2N with compound I can be described by the equation:
while extraction constant can be expressed as
The concentration constant of HTf2N extraction with compound I in dichloroethane was calculated from the dependence of ion Tf2N– distribution on compound I concentration in organic phase and Tf2N– and H+ ions in aqueous phase (Fig. 5). It was preliminary established that Li+ and Cl– ions do not transfer into organic phase under experimental conditions. The magnitude of \({{K}_{{{\text{HT}}{{{\text{f}}}_{{\text{2}}}}{\text{N}}}}}\) = 2690 ± 80 is much higher than the constant of HNO3 extraction with this compound (Table 1), which is due to much higher hydrophobicity of Tf2N– anion as compared with \({\text{NO}}_{3}^{ - }.\)
The value of synergic effect S = D/D0 (D and D0 are distribution ratios of REE(III) in the presence and in the absence of IL in organic phase) decreases when [HNO3] rises. Thus, upon extraction of Eu(III), the value of S decreases from 2150 to 2.2 when [HNO3] increases from 0.3 to 3 mol/L. The character of DLn–Z dependence for REE(III) extraction from nitric acid solutions with solutions of compound I in IL and dichloroethane considerably differs (Fig. 6). One can see that the extraction selectivity of heavy REE(III) in the system with IL is higher than on the use of dichloroethane as a solvent: the values of βLu/Sm in these systems are 70.5 and 6.0, respectively.
Stoichiometric ratio REE(III) : I in the complexes extracted in the presence of IL vary from 1 : 3 to 1 : 2 as HNO3 concentration in aqueous phase increases (Fig. 7), i.e., the system with IL displays the growth of solvation numbers in extracted complexes as compared with extraction with solutions of compound I in dichloroethane (Fig. 3). This is due to low coordination ability of Tf2N– ions [43], which seem to occupy outer coordination sphere of extracted complex, whereas REE(III) ions in the absence of IL are extracted with compounds I–III as coordination-solvated nitrates LnLs(NO3)3 (s = 1, 2) where \({\text{NO}}_{3}^{ - }\) ions coordinate to Ln3+ ions [36].
CONCLUSIONS
The presented data shows that the extraction ability of PP toward REE(III) increases in the series of compounds I < III < II. The efficiency and selectivity of REE(III) extraction with diethyl 2-[(diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate from nitric acid solutions considerably rises in the presence of ionic liquid, 1-butyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide, in organic phase.
REFERENCES
A. I. Mikhailichenko, E. B. Mikhlin, and Yu. B. Patrikeev, Rare Earth Metals (Metallurgiya, Moscow, 1987) [in Russian].
M. Yu. Alyapychev, V. A. Babain, and Yu. A. Ustynyuk, Russ. Chem. Rev. 85, 943 (2016). https://doi.org/10.1070/RCR4588
A. Leoncini, J. Huskens, and W. Verboom, Chem. Soc. Rev. 46, 7229 (2017). https://doi.org/10.1039/C7CS00574A
A. M. Rozen, Z. I. Nikolotova, and N. A. Kartasheva, Radiokhimiya 28, 407 (1986).
M. K. Chmutova, M. N. Litvina, G. A. Pribylova, et al., Radiokhimiya 41, 331 (1999).
H. T. Sartain, S. N. McGraw, and C. L. Lawrence, Inorg. Chim. Acta 426, 126 (2015). https://doi.org/10.1016/j.ica.2014.11.032
E. I. Goryunov, I. B. Goryunova, T. V. Baulina, et al., Ross. Khim. Zh. 54 (3), 45 (2010).
F. Arnaud-New, V. Bohmer, J. -F. Dozol, et al., J. Chem. Soc., Perkin Trans. 2, 1175 (1996). https://doi.org/10.1039/P29960001175
I. Smirnov, M. Karavan, V. Babain, et al., Radiochimica Acta. 95, 97 (2007). https://doi.org/10.1524/ract.2007.95.2.97
M. R. Yaftian, M. Burgard, C. Wieser, et al., Solvent Extr. Ion Exch. 16, 1131 (1998). https://doi.org/10.1080/07360299808934572
I. V. Smirnov, M. D. Karavan, T. I. Efremova, et al., Radiochemistry (Moscow, Russ. Fed.) 49, 482 (2007). https://doi.org/10.1134/S106636220705007
J. Kamenik, F. Sebesta, J. John, et al., J. Radioanal. Nucl. Chem. 304, 313 (2015). https://doi.org/10.1007/s10967-014-3543-x
E. M. Bond, U. Engelhardt, T. P. Deere, et al. Solvent Extr. Ion Exch. 15, 381 (1997). https://doi.org/10.1080/07366299708934484
S. Ouizem, D. Rosrio-Amorin, D. A. Dickie, et al., Dalton Trans. 43, 8368 (2014). https://doi.org/10.1039/C3DT53611D
H. Narita, T. Yaita, K. Tamura, and S. Tachimori, Radiochim. Acta 81, 223 (1998). https://doi.org/10.1524/ract.1998.81.4.223
Y. Sasaki, Y. Sugo, S. Suzuki, and S. Tachimori, Solvent Extr. Ion Exch. 19, 91 (2001). https://doi.org/10.1081/SEI-100001376
Z. -X. Zhui, Y. Sasaki, S. Suzuki, and T. Kimura, Anal. Chim. Acta 527, 163 (2004). https://doi.org/10.1016/j.aca.2004.09.023
Y. Sasaki, Y. Sugo, K. Morita, and K. L. Nash, Solvent Extr. Ion Exch. 33, 625 (2015). https://doi.org/10.1080/07366299.2015.1087209
E. Campbell, V. E. Holfeltz, G. B. Hall, et al. Solvent Extr. Ion Exch. 36, 331 (2018). https://doi.org/10.2172/1488863
S. A. Ansari, P. K. Mohapatra, A. Leoncini, et al., Dalton Trans. 46, 11355 (2017). https://doi.org/10.1039/C7DT03831C
A. N. Turanov, V. K. Karandashev, N. K. Evseeva, et al., Radiokhimiya 41, 219 (1999).
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Solv. Extr. Ion Exch. 17, 1423 (1999). https://doi.org/10.1080/07366299908934656
A. N. Turanov, V. K. Karandashev, V. E. Baulin, et al., Solv. Extr. Ion Exch. 27, 551 (2009). https://doi.org/10.1080/07366290903044683
A. M. Rozen, Z. I. Nikolotova, N. A. Kartasheva, and K. S. Yudina, Dokl. Akad. Nauk SSSR 222, 1151 (1975).
A. M. Safiullina, O. A. Sinegribova, V. E. Baulin, et al., Tsvetn. Metall., No. 3, 43 (2012).
M. Koel, CRC Crit. Rev. Anal. Chem. 35, 177 (2005). https://doi.org/10.1080/10408340500304016
Z. Kolarik, Solvent Extr. Ion Exch. 31, 24 (2013). https://doi.org/https://doi.org/10.1080/07366299.2012.700589
I. Billard, Handbook on the Physics and Chemistry of Rare Earths43, 213 (2013).
P. K. Mohapatra, Chem. Prod. Proc. Model. 10, 135 (2015).
K. Nakashima, F. Kubota, T. Maruyama, and M. Goto, Anal. Sci. 19, 1097 (2003). https://doi.org/10.2116/analsci.19.1097
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Russ. J. Inorg. Chem. 53, 970 (2008). https://doi.org/10.1134/S0036023608060272
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Radiochemistry 50, 266 (2008). https://doi.org/10.1134/S1066362208030090
K. Shimojo, K. Kurahashi, and H. Naganava, Dalton Trans. 37, 5083 (2008).
A. N. Turanov, V. K. Karandashev, and V. E. Baulin, Solv. Extr. Ion Exch. 26, 77 (2008). https://doi.org/10.1080/07366290801904871
S. Panja, P. K. Mohapatra, S. C. Tripathi, et al., Sep. Purif. Technol. 96, 289 (2012). https://doi.org/10.1016/j.seppur.2012.06.015
S. V. Demin, S. E. Nefedov, V. I. Zhilov, et al., Russ. J. Inorg. Chem. 57, 897 (2012). https://doi.org/10.1134/S0036023612060095
P. Bonhote, A. P. Dias, N. Papageorgiou, et al., Inorg. Chem. 35, 1168 (1996). https://doi.org/10.1021/ic951325x
A. M. Rozen and B. V. Krupnov, Russ. Chem. Rev. 65, 973 (1996). https://doi.org/10.1070/RC1996v065n11ABEH00021
V. S. Vlasov and A. M. Rozen, Radiokhimiya 30, 146 (1988).
K. B. Yatsimirskii, N. A. Kostromina, Z. A. Sheka, et al., Chemistry of Rare-Earth Complexes (Naukova Dumka, Kiev, 1966).
A. N. Turanov, V. K. Karandashev, and A. N. Yarkevich, Radiochemistry 60, 170 (2018). https://doi.org/10.1134/S1066362218020078
C. Gaillard, M. Boltoeva, I. Billard, et al., Chem Phys Chem 16, 2653 (2015). https://doi.org/10.1002/cphc.201500283
K. Binnemans, Chem. Rev. 107, 2593 (2007). https://doi.org/10.1021/cr050979c
Funding
This work was performed under the State Contract of 2019 for the Institute of Solid-State Physics, Russian Academy of Sciences (RAS), the Institute of Microelectronics Technology and High Purity Materials RAS, the Frumkin Institute of Physical Chemistry and Electrochemistry RAS, the Institute of Physiologically Active Substances RAS and financially supported in part by the Russian Foundation for Basic Research (project no. 18-29-24069) and the Ministry of Education and Science of the Russian Federation via the Program for the competitiveness enhancement of the National University of Science and Technology “MISIS” among leading worldwide research and education centers for 2013–2020 (no. K1-2014-026).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by I. Kudryavtsev
Rights and permissions
About this article
Cite this article
Turanov, A.N., Karandashev, V.K., Baulin, V.E. et al. Extraction of Rare-Earth Elements(III) from Nitric Acid Solutions with Diethyl 2-[(Diphenylphosphoryl)methoxy]-5-ethylphenylphosphonate. Russ. J. Inorg. Chem. 64, 1297–1303 (2019). https://doi.org/10.1134/S0036023619100164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023619100164