Abstract
Lipid and fatty acid modifications induced by the effects of various salinity patterns on the juvenile pink salmon Oncorhynchus gorbuscha (the Olkhovka River and the White Sea) within the experiment have been revealed. Concentrations of steroids, saturated fatty acids, and signaling molecules such as phospholipids (phosphatidylserine and phosphatidylinositol) and arachidonic acid under the hyperosmotic stress-related effects (keeping the fish for 1 h in the seawater after the time of transfer from the freswater) tend to increase. Decreases in phosphatidylcholine and n-6 saturated, monounsaturated, and polyunsaturated fatty acids are recorded in the juvenile pink salmon fish kept for 24 h in the seawater after the time of transfer them from the freswater, while the levels of phosphatidylethanolamine and n-3 polyunsaturated fatty acids (especially eicosapentaenoic and docosahexaenoic acids), on the contrary, tend to rise significantly. Lipid composition modifications in the juvenile pink salmon fish kept under the hypoosmotic stress conditions (24 h in freshwater after 24 h in seawater) induce stabilization of functioning the cell membrane structure, since the levels of bioeffectors including phosphatidylserine, phosphatidylinositol, and arachidonic, eicosapentaenoic, and docosahexaenoic acids tend to decrease. A reduced amount of lipids (triacylglycerols) stored as an energy reserve is shown for all the experimental fish groups. The outcomes indicate the high levels of adaptive potential of the juvenile pink salmon fish kept under the effects of abrupt salinity change in the water environments and their readiness for downstream migration not long before leaving the nests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Most fishes have variable salinity tolerance due to the mechanisms that control changes in the osmoregulatory strategy from active salt absorption to salt secretion and from water excretion to water retention (Kültz, 2015). However, salinity represents a critical environmental factor, which effects change the regulation of osmotic pressure and metabolism in hydrobionts (Marshall, 2002; Mancera and McCormick, 2007; Si et al., 2018). Within the responses of fish to variable salinities, somatotropic hormone secretion and transmembrane regulator proteins are first stimulated (receptors and ion channels) (Martins et al., 2014; Kültz, 2015; Li et al., 2017). Triggering these signaling mechanisms causes both changes in the structure and functions of some osmoregulatory organs (gills and kidneys) and reorganization of physiological processes associated with osmoregulation (Marshall, 2002; Arjona et al., 2007; Herrera et al., 2009; Vargas-Chacoff et al., 2015). In euryhaline fishes including the salmon fish, similar modofications at different levels of functional structure are adaptive evolutionary (McKenzie et al., 2001; Handeland et al., 2003).
The humpback salmon Oncorhynchus gorbuscha is assigned to the anadromous fish of early smoltification. It starts life in freshwater to migrate to the sea immediately after the yolk sac has been absorbed. The juvenile salmon fish migration to the marine environments is followed by significant modifications in morphology, behavior, and physiology (Folmar and Dickhoff, 1980; Mancera and McCormick, 2007). Transformation induces activation of neuroendocrine and endocrine systems, which causes the biochemical restructuring in the juvenile fish to prepare for a fresh-to-salt water migration (Wedermeyer et al., 1980). Pre-adaptive changes such as alterations in lipid metabolism are of special importance during smoltification, since they are associated with biological membrane restructuring, energy input changes, and synthesis of bioeffectors (Li and Yamada, 1992; Tocher et al., 1995).
The objective of the survey is to study the lipid composition modifications in the juvenile pink salmon fish within the experiment on transformational adaptation to changes in salinity of the water environments during fresh-to-salt water migrations of the analyzed specimens and vice versa.
MATERIALS AND METHODS
Experimental design. Juvenile pink salmon fish were sampled from the spawning nest found in the Olkhovka River (the White Sea basin; 66°14′36′′ N, 37°08′46′′ E) on June 20, 2019 and put them into the hatchery in the same river to start the experiment a day later (Fig. 1).
The fish in the hatchery were allocated into two groups. The first group (control, 10 specimens) was recorded for the subsequent biochemical analysis. The second group (experimental, >30 specimens) were put into the seawater tank (25‰ salinity) at the temperature of 14.6°С. Sampling the juvenile fish from the second group to record (10 specimens a sample) for studying was performed after 1 and 24 h of keeping them in the seawater. The rest fish kept in the seawater tank for 24 h were relocated into the freswater tank to sample 10 specimens after 24 h and record for a subsequent analysis. The biological material was fixed with the Folch mixture (chloroform/methanol extraction at 2 : 1 ratio in volume) to prepare one fish for each probe. Fish translocation from certain environmental conditions to the other environments was performed minimizing the stress-related effects in accordance with the methodical recommndations to conduct a survey (Guidelines …, 2014). The water in the juvenile fish tanks was kept aerated with the Sera Air 275 R compressor (Germany). The values for the fish total length (TL) and body weight are present in Table 1. There was no evidence of fish mortality during the experiment.
Lipid analysis. The parameters for the lipid profile in juvenile pink salmon fish have been studied. The lipid profile includes total lipids, total phospholipids, triacylglycerins, cholesterol, and cholesterol esters, individual phospholipids, and fatty acids of total lipids. The individual phospholipids consist of phosphatidylinositol, phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and lysophospahtidylcholine. The fatty acids of total lipids are composed of saturated fatty acids (SFA) including the palmitic acid (16:0), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA). The two N-3 and N-6 long chain PUFA families represent PUFA. The major identified members of these families are the eicosapentaenoic (20:5, N-3) and docosahexaenoic (22:6, N-3) acids and the arachidonic acid (20:4, N-6). Fatty acid designation includes the number of carbon atoms per molecule, the colon referred to a double bond number, and the series of the family of this fatty acid.
Lipid extraction from the fixed material was performed with the Folch method (Folch et al., 1957). Total lipids were separated at the room temperature with the method of ascending thin layer chromatography in a solvent system including petroleum ether : diethyl ether : acetic acid at a ratio of 90 : 10 : 1 in quantities, respectively (Stahl, 1965). The solvent component concentrations were determined with the spectrophotometric analyses (Sidorov et al., 1972; Engelbrecht et al., 1974). The analysis for certain phospolipid fractions was performed with the high performance liquid chromatography (HPLC). The mobile phase (eluent) consisted of a mixture of solvents including acetonitrile : hexane : methanol : phosphoric acid at a ratio of 918.0 : 30.0 : 30.0 : 17.5 in quantities, respectively. The eluent flow rate was 1.0 mL/min. Detection was performed with the UV spectrophotometer at 206 nm wavelength. The “MultiChrom Analytic Software, v. 1.5” (“Chromatek”, Russia) was used to analyze the chromatograms. Certain phospholipid fractions were identified following the standards as follows: phospholipid mixture for HPLC (Supelco, USA) and standard mixtures of phosphatidylserine, phosphatidylcholine, and phosphatidylinositol (Sigma, USA). In order to perform the fatty acid test, the separated lipids were subjected to the direct methylation (Tsyganov, 1971). The produced fatty acid methyl esters were separated with the “Crystal 5000” chromatographer (“Chromatek, Russia). Fatty acids were identified through both the comparison of completion time for peaks of experimental spsecimen and markers and the chain length-equivalent estimation to compare it with the table data (Jamieson, 1975). A quantitave analysis was performed with the Chromatek Analytic 3” software (“Chromatek”, Russia). The research surveys were carried out with the Collective Scientific Equipment Unit, Karelian Research Center, Russian Academy of Sciences.
Data analysis. The data were processed with the variational inference techniques for statistics (Eliseeva, 2007).
RESULTS
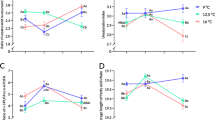
The concentration of total lipids in the juveniles spent 1 h in the seawater insignificantly increased, when compared to the control. With respect to the other experimental fish groups, the total lipid amount was not different from that in the control (Table 2). A reduced amount of triacylglycerols in the juvenile pink salmon fish over the period of the whole experiment was recorded. Concentrations of steroids (cholesterol and its esters) tended to increase significantly in the juveniles spent 1 h in the seawater after their transfer from the freswater. Therefore, the cholesterol/phospholipid ratio uncreased. Any changes in the percentage of phospholipids in the fish of all the experimental groups were not revealed.
In contrast to the total phospholipids, the dynamics of practically all the analyzed individual phospholipids excluding sphingomyelin and lysophospahtidylcholine were determined. A decrease in the phosphatidylcholine amount in the juvenile pink salmon fish (spent 1 and 24 h) under the effect of the seawater and an increase of this paramater for the juveniles after their salt-to-fresh water transfer were revealed. On the contrary, the dynamics of changing the phosphatidylethanolamine concentration in the juvenile pink salmon fish in the series of freshwater → seawater → freswater transfers were opposed to the phospahtidylcholine dynamics. The phosphatidylserine and phosphatidylinositol levels in the juvenile fish spent 1 h in the seawater were more than four times higher than that in the control (р ≤ 0.05), while the concentrations of these phospholipids deacreased, but not reverted to the control values, after the fish salt-to-freshwater transfer (р ≤ 0.05).
Among the saturated fatty acids, the palmitic acid accounted for the greatest proportion (16:0). Its amount was exactly what determined the SFA dynamics. Thus, the SFA level in the juvenile fish spent 1 h in the seawater significantly increased, while it dropped suddenly after 24 h, unchaging after the fish transfer into the freshwater. With respect to the control fish group, the MUFA accounted for the greater proportion among the other types of fatty acids, amounting to 46.71 ± 2.3% of their total sum (р ≤ 0.05). Under the effect of the seawater, the MUFA level in the juvenile pink salmon fish decreased, while it increased after the fish transfer into the freshwater. The seawater induced an increase in the PUFA level in the fish. However, the maximum n-6 PUFA level was observed in the fish spent 1 h in the seawater (due to the arachidonic acid). The maximum n-3 PUFA level (due to the eicosapentaenoic and
docosahexaenoic acids) was recorded in the juvenile pink salmon fish spent 24 hours in the seawater. As opposed to the control fish group (kept in freswater), the PUFA compound comprising 50% of the sum dominated the fatty acid composition in the juvenile pink salmon fish spent 24 h in the seawater. After a salt-to-fresh water transfer of the juveniles, a reduction in the PUFA amount was revealed. In addition, the arachidonic acid amount reduced most significantly, which was lower than the control level (Table 2).
DISCUSSION
Acclimation of euryhaline fishes to the variable salinity of water environments tends to induce the osmoregulatory changes in an organism to maintain homeostasis regulating the internal conditions within the phisiological optima and critical limits of functions. Biochemical mechanisms as the bases of predadaptation and smoltification play an important role in osmoregulation of salmon fishes especially at the level of lipid metabolism (Li and Yamada, 1992; Tocher et al., 1995).
Effective adaptation of fish to variable salinity is followed by increasing the energy input of an organism (Arjona et al., 2007; Soengas et al., 2007). Fatty acids are the main sources of energy. Therefore, a reduced amount of triacylglycerols in the fish over the period of the whole experiment is associated with activation of their hydrolisis to release the fatty acids involved in β-oxidation. Thus, the gilthead seabream Sparus aurata use triacylglycerols as energy resources in acclimation to the seawater (Sangiao-Alvarellos et al., 2005).
Euryhaline fish tolerance to osmotic stress is first caused by the specificity of a biophysical structure and lipid composition of cell membranes (Logue et al., 2000; Tocher et al., 2008). Initial cell-membrane injury induced by hyper- and hypoosmotic stress causes disorders in their dynamic response functins (Hansen et al., 2002). The secondary effects such as loss of membrane integrity increasing subsequent permeability may be followed by disorders in physiological homeostasis (tertiary effects), espicially relative to breathing and neuromascular coordination, which could be fatal (Hochachka and Somero, 2002). The adaptive evolutionary mechanisms in the euryhaline fishes allow them to respond rapidly to these adverse effects due to the dynamic modifications of structural lipids (steroids and phospholipids) and fatty acid compounds in tissues (Tocher et al., 2008).
It is common that cholesterol palys a key role in regulation of biological membrane permeability, influencing the microviscosity and lipid molecular mobility in a membrane (Kreps, 1981). This research survey has revealed a significant increase in the cholesterol amount and, consequently, an increase in the cholesterol/phospholipid ratio in the juvenile fish at the reference higher salinity, which may indicate a reduction in permeability of biological mambranes to ions. In addition to the ion transport, cholesterol regulates the water transport (Robertson and Hazel, 1999). Activation of synthesis of steroids including steroid hormones involved in the iono- and osmoregulatory mechanisms probably occurs under the hyperosmotic stress-related effects (Allen et al., 2011; Sarkheil et al., 2017; Nemova et al., 2021).
Adaptation to the conditions of changed salinity patterns can induce phospholipid metabolic alterations in fish (Babili et al., 1996; Zwingelstein and Bodennec, 1998). This experiment has revealed a decrease in the level of phosphatidylcholine in the fish after their fresh-to-salt water transfer and, on the contrary, its increase with the fish returns into the freshwater. In addition, it has been also revealed that the levels of phosphatidylserine and phosphatidylinositol in the juvenile fish spent 1 h in the seawater significantly increased, while the increased level of phosphatidylethanolamine was observed in the juveniles after 24 h in the seawater. The phospholipids indicated above are involved in signaling mechanisms, since they are the precursors of bioeffectors (Tocher et al., 2008). These changes represent a part of mechanisms of biochemical adaptation, which immediately respond to the osmotic stress and, probably, focuse on maintaining the optimal functioning membrane proteins under the conditions of the experiment (Bogdano and Doukhan, 1999). It should be noted that the levels of phosphatidylcholine and phosphatidylethanolamine in the juvenile pink salmon fish spent 24 h in the freshwate (after the seawater) become similar to those in the control fish group.
A fresh-to-salt water transfer of the juvenile pink salmon fish causes the hyperosmotic stress, which initially induces an increase in the density of biological membranes (Tocher et al., 2008). This experiment has proven that both an increased cholesterol concentration and a risen proportion of saturated fatty acids along with a reduced level of MUFA in the juvenile fish spent 1 h in the seawater indicate the lower membrane fluidity (Table 2). A subsequent decrease in the levels of these fatty acids in the fish spent 24 hours in the seawater may be associated with the use of them as the sources of energy input. Similar modifications of fatty acid compounds in some fish species were shown in the research surveys conducted earlier (Jana et al., 2006; Hunt et al., 2011).
Induction of PUFA synthesis occurs under the hyperosmotic stress-related effects (Daikoku et al., 1982), which is focused on the restoring the biological membrane fluidity and, conseqyently, normalization of the membrane protein functions (Bell et al., 1997; Logue et al., 2000). This sresearch survey shows a significant PUFA amount increase in the juvenile pink salmon fish spent 24 h in the seawater. The experiment has revealed changes in the levels of the physiologically significant long-chain PUFA such as arachidonic, eicosapentaenoic, and docosahexaenoic acids (Table 2). Rising levels of phospholipid arachidonic and docosahexaenoic acids in various juvenile tissues tend to increase the fish tolerance to the seawater salinity effect (Daikoku et al., 1982; Harel et al., 2001). PUFA including arachidonic and eicosapentaenoic acids with 20 carbon atoms are the substrate for eicosanoid synthesis (prostoglandins, leukotrienes, and tromboxanes), representing a wide range of mediators to perform regulatory functions (Wedermeyer et al., 1980; Tocher et al., 1995; Bell et al., 1997). It is common that prostoglandins are involved in the iono- and osmoregulatory mechanisms in fish gills and kidneys. Therefore, they are considered important for adaptation to salinity changes (Tocher and Sargent, 1987; Mustafa and Srivastava, 1989; Bell et al., 1997). This research survey revealed the differences in the dynamics between the concentrations of arachidonic and eicosapentaenoic acids in the juvenile pink salmon fish under the hyper- and hypoosmotic stress-related effects, which can indicate the change in the ratio of PGH2 prostoglandins sunthesized from them to PGH3 prostoglandins with multidirectional physiological effects.
CONCLUSIONS
Therefore, the lipid and fatty acid modifications induced by the effects of various salinity patterns on the juvenile pink salmon within the experiment have been revealed. The hyperosmotic stress (after spending 1 h in salt water) induces the steroid synthesis in juveniles.
Changes in the percentage of the phospholipid compounds under the hyperosmotic stress-related effects are multidirectional. Thus, a decrease in phosphatidylcholine amount and increases in the contents of minor phospholipids such as phosphatidylserine and phosphatidylinositol, functioning as bioeffectors, the members of the inositol phosphate system, are revealed. On the contrary, a transfer of juveniles into the freshwater after keeping them in the seawater tends to increase the phosphatidylcholine amount, which indicates restoring the membrane fluidity. After transferring the juvenile pink salmon fish into the seawater, an increase in the cholesterol-to-phospholopids ratio is recorded. It can indicate a membrane viscosity increase as a specific response of cells to the osmotically induced stress. Consequently, the biochemical adaptive mechanisms are activated to maintain homeostasis, since the PUFA proportion tends to increase in the juveniles kept in the seawater for 24 h. In addition, the effects of the salinity patterns on the levels of phisiologically significant fatty acids should be noted. The arachidonic acid amount significantly increases in the juvenile pink salmon fish after spending the first hour in the seawater, while it is sharply reduced after the fish transfer into the freshwater. On the contrast, the greatest proportional increases in the eicosapentaenoic and docosahexaenoic acids are recorded in the juveniles spent 24 hours in the seawater, while it tends to decrease after their transfer into the freshwater. The modifications revealed in the lipid and fatty acid compounds can indicate the effective acclimation of the juvenile pink salmon fish to different salinity patterns of the water environments.
REFERENCES
Allen, P.J., McEnroe, M., Forostyan, T., et al., Ontogeny of salinity tolerance and evidence for seawater-entry preparation in juvenile green sturgeon, Acipenser medirostris, J. Comp. Physiol. B, 2011, vol. 181, no. 8, pp. 1045–1062. https://doi.org/10.1007/s00360-011-0592-0
Arjona, F.J., Vargas-Chacoff, L., and Ruiz-Jarabo, I., Osmoregulatory response of Senegalese sole (Solea senegalensis, Kaup 1858) to changes in environmental salinity, Comp. Biochem. Physiol. A, 2007, vol. 148, no. 2, pp. 413–421. https://doi.org/10.1016/j.cbpa.2007.05.026
Babili, M.E., Brichon, G., and Zwingelstein, G., Sphingomyelin metabolism is linked to salt transport in the gills of euryhaline fish, Lipids, 1996, vol. 31, no. 4, pp. 385–392. https://doi.org/10.1007/BF02522924
Bell, J.G., Tocher, D.R., Farndale, B.M., et al., The effect of dietary lipid on polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation, Ibid., 1997, vol. 32, no. 5, pp. 515–525. https://doi.org/10.1007/s11745-997-0066-4
Bogdanov, M. and Doukhan, V., Lipid-mediated protein folding, Zh. Biol. Khim., 1999, vol. 274, pp. 827–830.
Daikoku, T., Yano, I., and Masuf, M., Lipid and fatty acid composition and their changes in the different organs and tissues of guppy Poecilia reticulata on sea water adaptation, Comp. Biochem. Physiol. A, 1982, vol. 73, no. 2, pp. 167–174. https://doi.org/10.1016/0300-9629(82)90050-0
Eliseeva, I.I., Statistika (Statistics), Moscow: Vyssh. Obrazovanie, 2007.
Engelbrecht, F.M., Mari, F., and Anderson, J.T., Cholesterol determination in serum. A rapid direction method, S. Afr. Med. J., 1974, vol. 48, no. 2, pp. 250–256.
Folch, J., Lees, M., and Stanley, G.H.S., A simple method for the isolation and purification of total lipides from animal tissues, J. Biol. Chem., 1957, vol. 226, no. 1, pp. 497–509. https://doi.org/10.1016/S0021-9258(18)64849-5
Folmar, L.C. and Dickhoff, W.W., The parr-smolt transformation (smoltification) and seawater adaptation in salmonids, Aquaculture, 1980, vol. 21, no. 1, pp. 1–37. https://doi.org/10.1016/0044-8486(80)90123-4
Guidelines for the Use of Fishes in Research, Bethesda: Am. Fish. Soc., 2014.
Handeland, S.O., Bjornsson, B.T., Arnesen, A.M., and Stefansson, S.O., Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and fanned strains, Aquaculture, 2003, vol. 220, nos. 1–4, pp. 367–384. https://doi.org/10.1016/S0044-8486(02)00508-2
Hansen, H.J.M., Kelly, S.P., Grosell, M., and Wood, C.M., Studies on lipid metabolism in trout (Oncorhynchus mykiss) branchial cultures, J. Exp. Zool., 2002, vol. 293, no. 7, pp. 683–692. https://doi.org/10.1002/jez.10166
Harel, M., Gavasso, S., Leshin, J., et al., The effect of tissue docosahexaenoic and arachidonic acids levels on hypersaline tolerance and leucocyte composition in striped bass (Morone saxatilis) larvae, Fish Physiol. Biochem., 2001, vol. 24, no. 2, pp. 113–123. https://doi.org/10.1023/A:1011924704459
Herrera, M., Vargas-Chacoff, L., Hachero, I., et al., Osmoregulatory changes in wedge sole (Dicologoglossa cuneata, Moreau, 1881) after acclimation to different environmental salinities, Aquac. Res., 2009, vol. 40, no. 7, pp. 762–771. https://doi.org/10.1111/j.1365-2109.2008.02147.x
Hochachka, P.W. and Somero, G.N., Biochemical Adaptation: Mechanism and Process in Physiological Evolution, New York: Oxford Univ. Press, 2002.
Hunt, A.O., Oxkan, F.E., Engin, K., and Tekelioglu, N., The effects of freshwater rearing on the whole body and muscle tissue fatty acid profile of the European sea bass (Dicentrarchus labrax), Aquacult. Int., 2011, vol. 19, no. 1, pp. 51–61. https://doi.org/10.1007/s10499-010-9340-9
Jamieson, G.R., GLS identification techniques for long-chain unsaturated fatty acids, J. Chromatogr. Sci., 1975, vol. 13, no. 10, pp. 491–497. https://doi.org/10.1093/chromsci/13.10.491
Jana, L., Huang, X., Zhang, L., and Zhuang, P Hematological parameters of Amur sturgeon, Acipenser schrencki, during different salinity domestication, Mar. Fish, 2006, vol. 28, pp. 177–184.
Kreps, E.M., Lipidy kletochnykh membran (Cell Membrane Lipids), Leningrad: Nauka, 1981.
Kültz, D., Physiological mechanisms used by fish to cope with salinity stress, J. Exp. Biol., 2015, vol. 218, no. 12, pp. 1907–1914. https://doi.org/10.1242/jeb.118695
Li, H.-O. and Yamada, J., Changes of the fatty acid composition in smolts of Masu salmon (Oncorhynchus masou), associated with desmoltification and seawater transfer, Comp. Biochem. Physiol. A, 1992, vol. 103, no. 1, pp. 221–226. https://doi.org/10.1016/0300-9629(92)90266-S
Li, S., He, F., Wen, H., et al., Low salinity affects cellularity, DNA methylation, and mRNA expression of igf1 in the liver of half smooth tongue sole (Cynoglossus semilaevis), Fish Physiol. Biochem., 2017, vol. 43, no. 6, pp. 1587–1602. https://doi.org/10.1007/s10695-017-0395-7
Logue, J.A., Howell, B.R., Bell, J.G., and Cossins, A.R., Dietary n-3 long-chain polyunsaturated fatty acid deprivation, tissue lipid composition, ex vivo prostaglandin production, and stress tolerance in juvenile Dover sole (Solea solea L.), Lipids, 2000, vol. 35, no. 7, pp. 745–755. https://doi.org/10.1007/s11745-000-0581-3
Mancera, J.M. and McCormick, S.D., Role of prolactin, growth hormone, insuline-like growth factor and cortisol in teleost osmoregulation, in Fish Osmoregulation, New York: Sci. Pub., 2007, pp. 497–515. https://doi.org/10.1201/9780429063909
Marshall, W.S., Na+, Cl−, Ca2+ and Zn2+ transport by fish gills: Retrospective review and prospective synthesis, J. Exp. Zool., 2002, vol. 292, no. 3, pp. 264–293. https://doi.org/10.1002/jez.10127
Martins, Y.S., Melo, R.M.C., Campos-Junior, P.H.A., et al., Salinity and temperature variations reflecting on cellular PCNA, IGF-I and II expressions, body growth and muscle cellularity of a freshwater fish larvae, Gen. Comp. Endocrinol., 2014, vol. 202, pp. 50–58. https://doi.org/10.1016/j.ygcen.2014.03.047
McKenzie, D.J., Cataldi, E., Romano, P., et al., Effects of acclimation to brackish water on tolerance of salinity challenge by young-of-the-year Adriatic sturgeon (Acipenser naccarii), Can. J. Fish. Aquat. Sci., 2001, vol. 58, no. 6, pp. 1113–1121. https://doi.org/10.1139/f01-058
Mustafa, T. and Srivastava, K.C., Prostaglandins (eicosanoids) and their role in ectothermic organisms, Adv. Comp. Environ. Physiol., 1989, vol. 5, pp. 157–207. https://doi.org/10.1007/978-3-642-74510-2_6
Nemova, N.N., Kaivarainen, E.I., Rendakov, N.L., et al., Cortisol content and Na+/K+-ATPase activity under adaptation of juvenile pink salmon Oncorhynchus gorbuscha (Salmonidae) to salinity changes, J. Ichthyol., 2021, vol. 61, no 5, pp. 771–778. https://doi.org/10.1134/S0032945221050118
Robertson, J.C. and Hazel, J.R., Influence of temperature and membrane lipid composition on the osmotic water permeability of teleost gills, Physiol. Biochem. Zool., 1999, vol. 72, no. 5, pp. 623–632. https://doi.org/10.1086/316699
Sangiao-Alvarellos, S., Arjona, F.J., and Martín del Río, M.P., Time course of osmoregulatory and metabolic changes during osmotic acclimation in Sparus auratus, J. Exp. Biol., 2005, vol. 208, no. 22, pp. 4291–4304. https://doi.org/10.1242/jeb.01900
Sarkheil, M., Sorki, M.P., and Raefipour, H., Effects of acclimation to seawater salinity on some blood parameters in wild Caspian brown trout, Salmo trutta caspius, Comp. Clin. Pathol., 2017, vol. 26, no. 6, pp. 1315–1318. https://doi.org/10.1007/s00580-017-2531-2
Shtal’, E., Khromatografiya v tonkikh sloyakh (Chromatography in Thin Layers), Moscow: Mir, 1965.
Si, Y., Wen, H., Li, Y., et al., Liver transcriptome analysis reveals extensive transcriptional plasticity during acclimation to low salinity in Cynoglossus semilaevis, BMC Genomics, 2018, vol. 19, Article 464. https://doi.org/10.1186/s12864-018-4825-4
Sidorov, V.S., Lizenko, E.I., Bolgova, O.M., and Nefedova, Z.A., Fish lipids. 1. Methods of analysis. Tissue specificity of vendace Coregonus albula L., in Lososevye (Salmonidae) Karelii (Salmon Fishes (Salmonidae) of Karelia), Petrozavodsk: Karel. Fil. Akad. Nauk SSSR, 1972, pp. 152–163.
Soengas, J.L., Sangiao-Alvarellos, S., Laiz-Carrio’n, R., and Mancera, J.M., Energy metabolism and osmotic acclimation in teleost fish, in Fish Osmoregulation, New York: Sci. Pub., 2007, pp. 277–308. https://doi.org/10.1201/9780429063909
Tocher, D.R. and Sargent, J.R., The effects of calcium ionophore A23187 on the metabolism of arachidonic and eicosapentaenoic acids in neutrophils from a marine teleost fish rich in (n-3) polyunsaturated fatty acids, Comp. Biochem. Physiol. B, 1987, vol. 87, no. 4, pp. 733–739. https://doi.org/10.1016/0305-0491(87)90381-6
Tocher, D.R., Castell, J.D., Dick, J.R., and Sargent, J.R., Effects of salinity on the fatty acid compositions of total lipid and individual glycerophospholipid classes of Atlantic salmon (Salmo salar) and turbot (Scophthalmus maximus) cells in culture, Fish Physiol. Biochem., 1995, vol. 14, no. 2, pp. 125–137. https://doi.org/10.1007/BF00002456
Tocher, D.R., Bendiksen, E.A., Campbell, P.J., and Bell, J.G., The role of phospholipids in nutrition and metabolism of teleost fish, Aquaculture, 2008, vol. 280, nos. 1–4, pp. 21–34. https://doi.org/10.1016/j.aquaculture.2008.04.034
Tsyganov, E.P., Method of direct lipid methylation after TLC without elution from silica gel, Lab. Delo, 1971, no. 8, pp. 490-493.
Vargas-Chacoff, L., Saavedra, E., Oyarzun, R., et al., Effects on the metabolism, growth, digestive capacity and osmoregulation of juvenile of Sub-Antarctic Notothenioid fish Eleginops maclovinus acclimated at different salinities, Fish Physiol. Biochem., 2015, vol. 41, no. 6, pp. 1369–1381. https://doi.org/10.1007/s10695-015-0092-3
Wedermeyer, G.A., Saunders, R.L., and Clarke, W.C., Environmental factors affecting smoltification and early marine survival of Anadromous Salmonids, Mar. Fish. Rev., 1980, vol. 42, no. 6, pp. 1–14.
Zwingelstein, G. and Bodennec, J., Phospholipid metabolism in euryhaline fish and crustaceans. Effects of environmental salinity and temperature, Recent Res. Dev. Lipids Res., 1998, vol. 2, pp. 39–52.
Funding
The research survey was carried out with a financial support of State Task FMEN-2022-0006 (State Registration no. 122032100052-8) for Institute of Biology, Karelian Research Center, Russian Academy of Sciences
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by O. Zhiryakova
Rights and permissions
About this article
Cite this article
Vasileva, O.B., Efremov, D.A., Ruokolainen, T.R. et al. Effects of Salinity on Lipid Composition in Juvenile Pinc Salmon Oncorhynchus gorbuscha (Salmonidae). J. Ichthyol. 63, 591–597 (2023). https://doi.org/10.1134/S0032945223030165
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945223030165