Abstract
The larvae and/or juveniles of coastal species were found in the composition of oceanic ichthyoplankton sampled during the 43rd expedition of R/V “Akademik Vavilov” in the southern waters of the North Atlantic. These fish included insufficiently-studied taxonomic forms from five families: Myripristis jacobus (Holocentridae), Ctenogobius sp., Gobionellus oceanicus, Gobioides grahamae (Gobiidae), Eleotris sp. 1., Dorminator maculatus (Eleotridae), Spariosoma sp. 1 (Scaridae), and Pontinus nematophthalmus, (Scorpaenidae). The paper presents illustrated descriptions of these forms and discusses the problems of their identification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Information on the larvae of many groups of fish from the open and coastal regions of the west-central North Atlantic is presented in the report by Richards (2005). However, despite the huge number of publications on the early stages of fish development, the larvae and juvenile specimens of many taxa have not yet been described; there are still problems in a number of species and families. To solve these problems, a detailed study on the morphology of the larvae and the identification of important diagnostic features and their variability in ontogenesis are necessary.
The present paper aims to fulfill existing gaps in the knowledge on the morphology of larvae of poorly studied coastal fishes from the families Holocentridae, Gobiidae, Eleotridae, Scaridae, and Scorpaenidae. Illustrated descriptions of the early stages of development in these species are presented.
MATERIALS AND METHODS
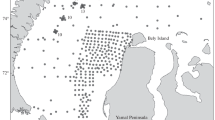
Early stages of fish sampled during the 43rd expedition of R/V Akademik Vavilov served as materials for the present paper. These materials were collected in the cross-section between 30° N and the equator in October 2016 (Fig. 1). The ichthyoplankton was sampled using Isaacs-Kidd midwater trawl in Samyshev-Aseev modification (MWT, length 25 m, knotless 5‑mm mesh net and apex insert of capron no. 15 insert, 6-m2 mouth area). The samples were preserved and stored in 4% formaldehyde. The larvae were stained with alizarin following the standard procedure. All illustrations given in the paper are original.
The following abbreviations for the features are used in the paper: SL—standard body length; с—head length; Н—maximal body height; Ha—body height above anus; h—minimal body height; hP—height of pectoral fin base; lP—length of pectoral fin rays; аА, aD—anteanal and antedorsal distances; ао—snout length; о—horizontal diameter of eye; io—interorbital distance; D (D1, D2) А, Р, V, C—number of rays in dorsal (first and second), anal, pectoral, pelvic and caudal fins, respectively; Br.—number of branchiostegal rays; sp. br.—number of gill rakers on the upper and lower parts of the first gill arch; vert.—number of vertebrae.
RESULTS AND DISCUSSION
Family Holocentridae
Myripristis jacobus (Fig. 2)—two larvae, SL 10.2 and 10.5 mm (sampling station 2665).
Description. D XI 14, A IV 13, P 14, V I 6, С (3 + 9) + (9 + 3), sp.br. 11+. Both larvae at “rhynchichthys” stage. Larva high-bodied, slightly teretial, body sharply tapering in caudal direction (h 7% SL). Maximal height (H 33–35% SL) at level of pectoral fins. Head massive, with serrated rostrum, forked at end (c 54–56% SL). Eyes very big, rounded (o 25–26% с). Head with large rostral, supraoccipital, supraopercular, third preopercular, and small fourth and fifth postorbital spines; all spines have serrated edges. Three serrated crests at each side of head above eye. Complete formation of rays in all fins. Border between D1 and D2 well visible owing to different lengths of rays. Dorsal fin starts slightly behind base of pectoral fin (aD 60–62% SL); anal fin, under vertical of soft rays' beginning in dorsal fins (aA 73% SL); pelvic fins situated under pectoral fins. Gill rakers not fully formed. Scale covers whole body. Larvae intensively pigmented except for rostrum (only a few melanophores at end), jaws, preoperculum, fins (except for spiny rays of dorsal fin), caudal peduncle, and ventral body side.
Comparative notes. Based on the structure of swim bladder and ear capsule and connection between them, the family is divided into two subfamilies: Holocentrinae and Myripristinae (Nelson, 1955). The early developmental stages of these subfamilies differ in the structure of the rostral spine: in the Holocentrinae, there is a single rostral spine while the rostral spine in Myripristinae is bifurcate at the end (Lyczkowski-Shultz et al., 2000). Several larvae of Myripristinae are described from the waters of the Pacific Ocean: Myripristis spp. (Jones and Kumaran, 1962; Leis and Rennis, 1983) and M. leiognathos (Watson, 1996). Among the members of this subfamily that inhabit the waters of the central West Atlantic, only M. jacobus has meristic features indicated for our larvae: in Corniger and Plectrypops, there are more rays in the pectoral fins (16–18 vs. 14–15), in Ostichthys trachypoma, there are 12 spiny rays in the dorsal fin and 10–12 soft rays in the anal fin vs. 12–13 in M. jacobus (Kotlyar, 1996). Myripristis jacobus is an endemic of the tropical Atlantic and the only Atlantic species of the genus (Greenfield, 1968). The literature contains an illustrated description of this species' larvae SL 5.0 mm from the coastal waters of southern Brazil (Bonecker and Castro, 2006). The larvae are poorly pigmented and melanophores are found only on the lateral surface of the abdomen. It can be assumed (due to the lack of a complete series of larvae) that the color intensity increases significantly with increasing body size. Therefore, we identify our larvae with that previously described by Bonecker and Castro (2006).
Family Scorpaenidae
Pontinus nematophthalmus (Fig. 3), one larva SL 7.8 mm (sampling station 2674).
Description. D XII 9, A III 5, P 15, V I 5, С (5 + 8) + (7 + 5), sp.br. 4 + 10, Br. 7, 24 myomeres. Larva with high body, flattened laterally, gently tapering in caudal direction, greatest height at pectoral fins’ level (H 43.8% SL). Head massive, wide (c 48.7% SL, io 36.8% SL), upper profile of head with slight deflection. Eyes large, diameter of ~1/3 c. Head spines include large second, third, and fourth posterior preopercular, postorbital, and occipital spines; all spines with serrated edges; small first lower and upper suborbital, supraorbital, upper, and lower opercular spines, fifth posterior preopercular. Definitive number of rays present in all fins. First dorsal fin starts practically immediately after upper edge of head (aD 40.3% SL), while anal fin under middle of dorsal fin (aA 63.3% SL). Anus located slightly behind middle of body. Pectoral fin lobes large (lP 25% SL, hP 14.2% SL) pterygoid, 15 rays formed in them (given that ossification of rays in pectoral fins of scorpionfish larvae occurs at SL ~ 4–5 mm, their number can be considered definitive). Larva poorly pigmented: internal melanophore behind base of anal fin, membrane between fourth to sixth spiny ray of dorsal fin pigmented, three diagonally located spots on pectoral fin.
Comparative notes. The larva has head spines and meristic features typical for the species of fam. Scorpaenidae. By analogy with the Pacific larvae (Moser et al., 1977), we classify our larva to Pontinus based on a range of larval characteristics, namely: nonpigmented dorsolateral side of intestine (such a pigment is present in Scorpaena larvae), pterygoid pectoral fin (fan-shaped in Scorpaenodes and Scorpaena), its color (pigment on the distal margin of the fin is characteristic of Scorpaenodes larvae, some Pontinus and Scorpaena; continuous covering of the greater part of the fin for some Scorpaena, while a diagonal strip for some Pontinus), its length and height of the base. If we judge by the meristic features (sp. br. 4 + 10, Р 15, number of myomeres 24), such an identification seems justified. Adult individuals, at most six species of this genus, inhabit the coastal waters of northern South America: P. castor, P. helena, P. longispinis, P. nematophthalmus, P. rathbuni, and P. corallinus; the last four are most likely to be found in our sampling area (Eschmeyer, 1969; Buckup et al., 2003). All these species are similar in meristic features, the difference is observed only in the rays of the pectoral fin: such a small number (15) is characteristic only of P. nematophthalmus (15–17, usually 16) (Eschmeyer, 1969). The larvae of only one species are described (P. rathbuni), and they differ from ours in the character of pigmentation (Sanchez and Acha, 1988). It is worth noting that there are many descriptions of undetermined scorpion fish larvae SL <10 mm in the papers (Richards, 1990). This is due to the fact that the larvae of scorpionfish undergo certain morphological changes with growth, moreover, their coloring is very different from both the juveniles and the adult; therefore, it is practically impossible to correlate them. In this regard, our identification should be considered preliminary.
Family Gobiidae
Ctenogobius sp. (Fig. 4), 90 larvae SL 5.5–7.3 mm (sampling stations 2649-2, 2664, 2665, 2669, 2674, 2675).
Description. D1 V+, D2 12, A 13, P 16–17, V-, C (8 + 7)–(6 + 7), vert. 10 + 16 = 26, Br. 5. Larvae long, low-bodied, strongly flattened laterally (Ha 14–17% SL, h 5–6% SL), with small head (c 22–26% SL). Snout short (ao 20–22% c), eyes slightly oval (o 24–28% c). Jaws short, barely reaching vertical of eye’s front edge. First dorsal fin begins at level of fourth myomere (aD 34–37% SL); Second dorsal and anal fins located oppositely and begin at midbody level (aA 51% SL). In larvae, number of rays in unpaired and pectoral fins definitive (formation of rays in D1 not completed), pelvic fins been formed yet. Intestine relatively straight, anus opens at midbody level.Larvae poorly pigmented: several small melanophores on theanterolateral part of the lower jaw, unpaired pigment spot on dorsal side of terminal intestine section, several deep melanophores on caudal peduncle ventral side immediately behind anal fin; dorsal and posterior sides of swim bladder pigmented.
Comparative notes. The Ctenogobius and Gobioides larvae are the only representatives of the family in which both dorsal and posterior sides of the swim bladder are pigmented (Yeung and Ruple, 2006). However, there are more rays in the unpaired fins (D1 VII, D2 14–15, A I 13–14) in Gobioides. In our sampling area, the larvae of early developmental stages of Ctenogobius boleosoma, C. smaragdus, C. saepepallens, C. stigmaticus, C. shufeldti, and С. thoropsis may be present. The first two species, C. boleosoma and C. smaragdus, usually have D2 11 and A 12 vs. D2 12 and A 13 in the larvae we sampled as well as in C. stigmaticus, C. shufeldti, and C. saepepallens. The larvae of the latter three species have already been described (Wyanski and Targett, 2000; Baldwin and Smith, 2003). No transforming specimens were available for our study. This is why the identification is possible only to the genus.
Gobionellus oceanicus (Fig. 5), three larvae SL 8.5–11.2 mm (2665).
Description. D1 V+, D2 14, A 15, P 16–17, V-, C (8 + 7)–(6 + 7), vert. 10 + 16 = 26, Br. 5. Larvae fusiform (Ha 11–12% SL, h 5–6% SL) with rather small head (c 18–21% SL). Snout short (ao 17–21% c), eyes round, small (o 17–20% c). Jaws short, not reaching vertical of front edge of eye. First dorsal fin begins at beginning of second third of body (aD 34–35% SL). Second dorsal and anal fins located oppositely and start slightly behind middle of body (aA 52–56% SL); ventral fins located under pectoral fins. In caudal, anal, second dorsal, and pectoral fins, formation of rays complete. Five rays in first dorsal fin; formation of rays in it takes the longest time; in pelvic fin formation of rays not completed. Intestine relatively straight, anus opens at midbody level. Body of larva practically devoid of pigment: only internal point melanophores covering dorsal and back sides of swim bladder.
Comparative notes. Among the gobies dwelling in the coastal waters of Brazil, corresponding meristic features (D2 14, A 14–15, P 15–18, vert. 10 + 16 = 26) are characteristic of Gobioides grahamae and Gobionellus oceanicus (Murdy, 1998; Yeung and Ruple, 2006). The illustrated descriptions of G. oceanicus larvae SL 10.1–12.1 mm are available in the published papers (Wyanski and Targett, 2000; Baldwin and Smith, 2003). When we consider both plastic features and characteristics of pigmentation, the larvae from our samples do not differ from those described earlier. In terms of habitus, our larvae are very similar to Gobioides broussoneti (pigmentation is also present only above the swim bladder), but the differences in the meristic features are strong (D2 15, A 16, P 17–20, vert. 10 + 17 = 27). This is why we identify the larvae of that type as G. oceanicus.
Gobioides grahamae (Fig. 6)—51 larvae SL 7.0–10.0 mm (sampling stations 2649–2, 2669, 2674, 2675).
Description. D1 V+, D2 14, A 15, P 17, V-, C (8 + 7)–(6 + 7), vert. 10 + 16 = 26, Br. 5. Larvae fusiform (Ha 12–13% SL, h 6% SL) with rather small head (c 20–22% SL). Snout short (ao 17–21% c), eyes round, small (o 23–25% c). Jaws short, not reaching vertical of front edge of eye. First dorsal fin begins at level of fourth myomere (aD 34–35% SL). Second dorsal and anal fins located oppositely and start slightly behind middle of body (aA 52–54% SL); pelvic fins under pectoral fins. In caudal, anal, second dorsal, and pectoral fins, formation of rays complete. In first dorsal fin, five rays; in pelvic formation of rays just beginning. Intestine relatively straight, anus opens at midbody level. Pigment present only on dorsal and posterior sides of swim bladder.
Comparative notes. In terms of meristic features and character of pigmentation, the larvae do differ both from those we described and those descriptions of G. oceanicus larvae presented in the published papers (Wyanski and Targett, 2000; Baldwin and Smith, 2003). At the same time, there are differences concerning plastic features in the larvae of similar size (SL ~ 8 mm): body height (Ha 12–13% SL in our larvae vs. 11–12% SL in G. oceanicus) and eye diameter (23–25 vs. 17% SL). Besides G. oceanicus, only Gobioides grahamae has such a combination of meristic traits; moreover, there is a description in the literature of the early stages of development of a close species, Gobioides broussoneti, which, in addition to the meristic features, does not differ in habitus from the larvae we sampled.
Family Eleotridae
Eleotris sp. 1 (Fig. 7), 15 larvae SL 6.0–11.6 mm (sampling stations 2664, 2668, 2674, 2675).
Description. D1 V+, D2 I 8, A I 7–8, P 12–14, V 3+, C (9 + 7)–(8 + 9), vert. 25–26, Br. 6. Larvae long, low-bodied (H 13–15% SL) with relatively small head (c 23–26% SL). Snout short (ao 23–27% c), eyes oval (o 16–18% c). Jaws short, barely reaching vertical of eye’s front edge. First dorsal fin begins at level of fifth myomere (aD 36–41% SL). Second dorsal and anal fins located oppositely and start somewhat behind body’s middle (aA 53–58% SL).
In larva SL 11.6 mm, all rays in pectoral fins already formed; formation just starts in pelvic fin. Larvae with several small melanophores on anterolateral part of mandibule, melanophore on mandibule corner, supraocular and subocular branched melanophores situated closely to pigmented part of eye, internal point melanophores around ear section of neurocranium. Dorsal row of 7–10 melanophores from isthmus to vertical of D1 end; melanophore on dorsal part of terminal section of intestine. Subcaudal internal row of 9–11 melanophores formed by paired melanophores situated along base of anal fin; between anal and caudal fins, row turns to unpaired. From this unpaired row, several point melanophores form vertical dorsally-directed rows. Dorsal side of swim bladder and end of caudal peduncle pigmented.
Comparative notes. A similar pattern of pigmentation (the presence of pigment above the ear capsule of the brain and at the end of the caudal peduncle, on the dorsal side of the swim bladder, abdominal and subcaudal rows of melanophores) is characteristic of the larvae of Eleotris spp. and Erotelis smaragdus (Baldwin and Smith 2003; Maeda and Tachihara, 2005; Yeung and Ruple, 2006). However, the latter two forms differ from the larvae we sampled by the number of rays in the dorsal and anal fins (D2 11, A 10 vs. D2 9, A 9). Adult specimens of all four Eleotris species live in the waters of the central western Atlantic: E. amblyopsis, E. pisonis, E. beliziana, and E. perniger; they differ in the patterns of the location of the sensory papillae on the head. Larvae of E. pisonis and E. spp. (E. amblyopsis or E. pisonis by: Baldwin and Smith, 2003) are described, and the larvae from our samples differ slightly from E. pisonis by the lack of pigment at the distal edge of the upper jaw (E. spp. has it). In all likelihood, similarly with the larvae of Pacific Eleotris, the interspecific differences in the larvae concern the pattern of pigmentation of the end of caudal peduncle, the head, and the number of melanophores in the lower caudal row (Maeda and Tachihara, 2005). To identify these signs, a much larger number of individuals is necessary for analysis. For this reason, and due to the fact that diagnostic signs characteristic of adult individuals are absent in the larvae of our samples, identification to the species is impossible.
Dorminator maculatus (Fig. 8), six larvae, SL 6.0–7.3 mm (sampling stations 2665, 2675).
Description. D1 V+, D2 9, A 9–10, P 12–14, V 3+, C (9 + 7)–(8 + 9), vert. 25–26, Br. 6. Larvae fusiform (H 12–15% SL) with relatively small head (c 21–25% SL). Snout short (ao 21–24% c), eyes oval (o 17–20% c). Jaws short, not reaching vertical of eye’s front edge. First dorsal fin begins at level of fourth myomere (aD 38–39% SL). Second dorsal and anal fins located oppositely and start somewhat behind middle of body (aA 57–60% SL). In SL 7.3 mm larvae, ten rays formed in pectoral fins, pelvic ones not yet formed. Larvae with several small melanophores on anterolateral part of mandibule, ventral row of six to eight melanophores from isthmus to vertical of first dorsal fin’s end. Along base of anal fin, melanophores on both sides; subcaudal row becomes unpaired immediately behind anal fin. From this unpaired series, several point melanophores form vertically arranged strips. Dorsal side of swim bladder pigmented.
Comparative notes. In the described larvae, there is no pigmentation of the ear capsule and the end of the caudal peduncle, which is characteristic of Dorminator larvae (Yeung and Rouple, 2006). According to the latest data (Kullander, 2003), only D. maculatus can be met in the area where we caught the larvae. An illustrated description of this species larvae SL 8.1 and 22.1 mm is available in the literature (Ruple, 1984); the larvae from our samples show significant similarity both in meristic features and in the pigmentation pattern, differing only in the absence of melanophore at the corner of the low jaw.
Family Scaridae
Spariosoma sp. 1 (Fig. 9), ten larvae, SL 7.5–7.6 mm (sampling stations 2668, 2669, 2674, 2675).
Description. D IX 10, A III 10, P 13, C (3 + 7)–(6 + 4), vert. 9 + 16 = 25. Larvae rather low-bodied (H 13% SL, h 9% SL), strongly flattened laterally, with small head (c 21–22% SL). Snout short (ao 25% c), eyes oval (o 19–20% c) with pigmented choroid tissue at lower edge of eye. Jaws short, barely reaching vertical of eye’s front edge, teeth absent. Dorsal fin begins somewhat behind vertical of pectoral fin’s beginning (aD 28–30% SL); anal fin begins slightly behind the midbody (aA 57% SL). In all larvae, number of rays in unpaired and pectoral fins definitive, but pelvic fins not yet formed. Intestine straight, anus opens at level of body’s middle. Pigmentation of larvae consists of paired melanophore, located laterally on cardiac region (at level of pectoral fin’s base), subcaudal series of 13 internal melanophores and as pigmented dorsal side of swim bladder.
Comparative notes. In the west-central Atlantic, there are at least 16 species belonging to four genera of this family. By meristic features, these species show significant similarity; only representatives of Scarus have a greater number of rays in the pectoral fins (13–16 vs. 13 in the other species). At the same time, the larvae of this family differ well to the genus rank in terms of pigmentation: Spariosoma larvae have a melanophore on the body under the base of the pectoral fin, Scarus has pigment on the dorsal side of the caudal peduncle, Cryptotomus roseus has neither one nor the other pigment. By the presence of melanophore under the pectoral fin, we identify these larvae as Spariosoma. Of the seven species of this genus living in the sampling area, the early stages of the three species are described: S. atomarium, S. radians, and S. rubripinne; they differ in the location of erythrophores (Baldwin, 2013) but do not differ in the arrangement and number of melanophores. Because we do not have the successive stages of development, identification to the species levelis difficult.
REFERENCES
Baldwin, C.C., The phylogenetic significance of color patterns in marine teleost larvae, Zool. J. Linn. Soc., 2013, vol. 168, no. 3, pp. 496–563. https://doi.org/10.1111/zoj.12033
Baldwin, C.C. and Smith, D.G., Larval Gobiidae (Teleostei: Perciformes) of Carrie Bow Cay, Belize, Central America, Bull. Mar. Sci., 2003, vol. 72, pp. 639–674.
Bonecker, A.C.T. and Castro, M.S., Atlas de Larvas de Peixes da Região Central da Zona Econômica Exclusiva Brasileira, Rio de Janeiro: Museu Nacional, 2006.
Buckup, P.A., Figueiredo, J.D., and Moura, R.D., Catálogo das Espécies de Peixes Marinhos do Brasil, São Paulo: Mus. Zool., Univ. São Paulo, 2003.
Early Stages of Atlantic fishes: an identification guide for the Western Central North Atlantic, Richards, W.J., Ed., Boca Raton, FL: CRC Press, 2005. https://doi.org/10.1201/9780203500217
Eschmeyer, W.N., A systematic review of the scorpionfishes of the Atlantic Ocean (Pisces, Scorpaenidae), Occas. Pap. Calif. Acad. Sci., 1969, vol. 79.
Greenfield, D.W., The zoogeography of Myripristis (Pisces: Holocentridae), Syst. Biol., 1968, vol. 17, no. 1, pp. 76–87. https://doi.org/10.1093/sysbio/17.1.76
Jones, S. and Kumaran, M., Notes on eggs, larvae and juveniles of fishes from Indian waters. XII. Myripristis murdjan. XIII. Holocentrus sp., Indian J. Fish., A, 1962, vol. 9, no. 1, pp. 155–167.
Kotlyar, A.N., Beriksoobraznye ryby Mirovogo okeana (Beryciform Fishes of the World Ocean), Moscow: VNIRO, 1996.
Kullander, S.O., Gobiidae (Gobies), in Checklist of the Freshwater Fishes of South and Central America, Reis, R.E., Eds., Porto Alegre: Edipucrs, 2003, pp. 657–665.
Leis, J.M. and Rennis, D.S., The Larvae of Indo-Pacific Coral Reef Fishes, Sydney: N.S. Wales Univ. Press, 1983.
Lyczkowski-Shultz, J., Konieczna, M., and Richards, W.J., Occurrence of the larvae of beryciform fishes of the Gulf of Mexico, Bull. Sea Fish. Inst., 2000, vol. 3, no. 151, pp. 55–66.
Maeda, K. and Tachihara, K., Recruitment of amphidromous sleepers Eleotris acanthopoma, Eleotris melanosoma, and Eleotris fusca into the Teima River, Okinawa Island, Ichthyol. Res., 2005, vol. 52, no. 4, pp. 325–335. https://doi.org/10.1007/s10228-005-0289-z
Moser, H.G., Ahlstrom, E.H., and Sandknop, E.M., Guide to the Identification of Scorpionfish Larvae (Family Scorpaenidae) in the Eastern Pacific with Comparative Notes on Species of Sebastes and Helicolenus from Other Oceans, NOAA Technical Report NMFS CIRC vol. 402, Seattle: Natl. Ocean. Atmos. Admin., 1977.
Murdy, E.O., A review of the gobioid fish genus Gobioides, Ichthyol. Res., 1998, vol. 45, no. 2, pp. 121–133. https://doi.org/10.1007/BF02678554
Nelson, E.M., The morphology of the swim bladder and auditory bulla in the Holocentridae, Fieldiana Zool., 1955, vol. 37, pp. 121–137
Richards, W.J., List of the Fishes of the Western Central Atlantic and the Status of Early Life Stage Information, NOAA Technical Memorandum NMFS-SEFC vol. 267, Seattle: Natl. Ocean. Atmos. Admin., 1990.
Ruple, D., Gobioidei: development, in Ontogeny and Systematics of Fishes, Moser, H.G., et al., Eds., Am. Soc. Ichthyol. Herpetol. Spec. Publ. vol. 1, Lawrence, KZ: Am. Soc. Ichthyol. Herpetol., 1984, pp. 582–587.
Sanchez, R.P. and Acha, E.M., Development and occurrence of embryos, larvae and juveniles of Sebastes oculatus with reference to two Southwest Atlantic Scorpaenids: Helicolenus dactylopterus lahillei and Pontinus rathbuni, Meeresforsch. Rep. Mar. Res., 1988, vol. 32, pp. 107–133.
Watson, W., Holocentridae, in The Early Stages of Fishes in the California Current Region, California Cooperative Oceanic Fisheries Investigations, Moser, H.G., Ed., La Jona, CA: Southwest Fish. Sci. Center, 1996, no. 33, pp. 687.
Wyanski, D.M. and Targett, T.E., Development of transformation larvae and juveniles of Ctenogobius boleosoma, Ctenogobius shufeldti, and Gobionellus oceanicus (Pisces: Gobiidae) from western North Atlantic estuaries, with notes on early life history, Bull. Mar. Sci., 2000, vol. 67, no. 2, pp. 709–728.
Yeung, C. and Ruple, D., Gobiidae: Gobies, in Early Stages of Atlantic fishes: an identification guide for the Western Central North Atlantic, Richards, W.J., Ed., Boca Raton, FL: CRC Press, 2006, vol. 2, pp. 2029–2077.
Funding
The study was supported by the state assignment of Institute of Oceanology, Russian Academy of Sciences, project no. 0149-2019-0009 and financially supported by the Russian Science Foundation, project no. 19-14-00026.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Pavlov
Rights and permissions
About this article
Cite this article
Bolshakova, Y.Y., Evseenko, S.A. Ichthyoplankton of the Southern Waters of the North Atlantic: 1. Morphology in Poorly-Studied Larvae of Coastal Species. J. Ichthyol. 59, 689–696 (2019). https://doi.org/10.1134/S0032945219050011
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945219050011