Abstract—
The results of the analysis of the bolus of the larval river lamprey Lampetra fluviatlis caught in the Chernaya River in May are presented. A quantitative analysis of the main food components is performed; the taxonomic composition of algae and invertebrates is determined. The content of the digestive tract consists mainly (>90% of weight) of sand and detritus as well as algae and invertebrates. A large amount of diatoms, other plankton, and periphyton unicellular algae and coenobia, as well as plankton rotifers, have been first found among food items. It is shown that organisms from the river flow may play a critical role in larval lamprey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The study on feeding habits makes it possible to provide important information about lifestyle, trophic relations, and the role of organisms in the ecosystem. The data on feeding of ammocoetes are scanty and mainly concern the general information on qualitative composition of the bolus. The intestine content of ammocoetes was first described for the Lethenteron appendix (Creaser and Hann, 1929); the researchers detected a large amount of sand and plant residues and demonstrated that diatoms (Bacillariophyta) and desmids (Desmidiales) are the food items of ammocoetes.
The researchers usually indicate that ammocoetes are filter feeders and feed on detritus, which is comprised of plant and animal residues, bottom algae, mostly diatoms (Hardisty and Potter 1971; Moore and Mallatt, 1980; Makeeva et al., 2011; Loshakova and Knizhin, 2015). Meiobenic animals are also reported: small mollusks, chironomid larvae (Chironomidae), amphipods (Amphipoda), nematodes (Nematoda), sometimes bottom rotifers (Rotifera) (Moore and Potter, 1976; Nazarov, 2012). Most publications do not present a taxonomic list of organisms, which makes impossible the comparison of the food composition of larvae of one species in different seasons from different biotopes within one water body or from different rivers and the interspecies analysis.
In addition, it is difficult to determine the belonging of food objects either to bottom or plankton forms in the absence of taxonomic lists. The absence of the quantitative data also makes the comparison difficult and does not allow estimating the role of some components in the diet of ammocoetes.
In some publications, the role of an organic component of the detritus in the diet (Sutton and Bowen, 1994; Bowen et al., 1998) and matter of autochthonous and allochthonous origin (Hollet, 1995; Evans and Bauer, 2016) is reported. Experimental studies on feeding of larval lampreys demonstrated the ability of ammocoetes to consume different food components and determined assimilability of some of its components (Moore and Potter, 1976; Mallatt, 1983; Bowen et al., 1998; Jolley et al., 2012).
The purpose of this study is to make qualitative and quantitative analysis of the composition of the bolus of larval European river lamprey Lampetra fluviatilis and to determine the ecological affinity of the organisms, the components of the bolus.
MATERIALS AND METHODS
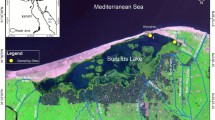
The material was collected at a distance of 5.9 km from the mouth of the Chernaya River inflowing the Gulf of Finland in the Baltic Sea (60°13′15.74″ N, 29°30′56.26″ E). Water temperature was 16–17°C. Larvae were sampled with a Kinalyov net at noon in a typical habitat of silty sediments covered with a detritus layer near a steep bank. A total of 39 lamprey larvae were caught, feeding was analyzed in 30 specimens with a total length (TL) of 16–109 mm. Samples of sediments and water above the sediments were collected, and 100 L of water from the main flow were filtered through an Upstein net (gauze no. 70). All samples were fixed in 4% formaldehyde.
Feeding was analyzed according to the common methods (Instruktsiya…, 1971; Metodicheskie rekomendatsii…, 1984). After determination of length and weight of the body of lamprey larvae, the intestine was dissected, extracted, and weighted. The degree of the gut fullness was visually estimated using the 6‑point scale of Lebedev (1936). The digestive tube in ammocoetes is hardly differentiated into sections; therefore, it was visually divided into three parts, an anterior with the esophagus, middle, and posterior sections. Each section was studied separately. The content was washed out and fixed in 4% formaldehyde. The weight of the bolus was determined as the difference in the weight of full and empty digestive tubes. Weighing of samples was performed using an OHAUS Discovery analytical balance to an accuracy of 0.1 mg.
Components of the bolus were sorted under an MBS 9 binocular microscope at 4 × 8 magnification distinguishing large inclusions, such as different vegetative remains and parts of large animal organisms. Solitary organisms in the intestine were detected and counted under a microscope at 10 × 10 magnification. The number of cells and mass of algae in the bolus were calculated according to the standard method (Metodicheskie rekomendatsii…, 1984; Radchenko et al., 2010). The bolus diluted with water was stirred up, and from 1/4 to 1/20 of its part depending on the amount of algae was selected for further analysis (Spetnitskaya et al., 2008). Single cells were counted under Mikromed 1 or Leica DM 1000 microscopes at 10 × 40 magnification. Three replicates were taken from each sample. The organisms were identified to the genus or species. The quantitative estimation of blue-green algae (Cyanobacteria) and spherical algae of the class Protococcophyceae was not made because of the complexity of identification and separation of some forms and their counts. The cell size for calculation of the volume and conversion into mass was determined using Leica LAS EZ software (Radchenko et al., 2010). The body mass of nematodes was calculated according to the formula W = 1.024L2.21 (Tsalolikhin, 1981); the body mass of rotifers and crustaceans was determined according to the formula W = qLb, where values of the q coefficient for each species were taken from literature sources (Alimov, 1989). The cluster analysis was used to assess the similarity of the bolus contents in different larvae; the Euclidean distance was a measure of similarity/dissimilarity. The secondary matrices were analyzed by the Ward method. The results of clustering are presented as dendrograms.
The statistical analysis of the data was carried out using Microsoft Office Excel 2010, PAST, and Statistica 10 software.
RESULTS
Characteristic of larval lamprey. According to the results of our studies on lamprey from the Chernaya River (Pavlov et al., 2014, 2017), ammocoetes are characterized by a nighttime dispersion; larvae burrow into the sediments at the morning twilight. In the present work, we consider ammocoetes extracted from sediments. Samples were collected during the spawning period of lamprey and fingerlings were still absent, so the sample (39 specimens) was comprised of 1‑year-old and older specimens. The proportion of ammocoetes of size class I (TL 16–29 mm) constituted 33% of the total number of sampled larvae, 18% of class II (30–49 mm), 26% of class III (50–69 mm), 18% of class IV (70–89 mm), and 5% of class V (90–109 mm). Thus, all size classes of ammocoetes represented in the river during that period of the year were recorded in the biotope.
The microbiotope structure. Ammocoetes were caught in a typical habitat for them, in the so-called ammocoete bed. The biotope was a river part near a steep bank. The water column thickness above the site was 15–70 cm, the thickness of the debris composed of large vegetative remains was 10–20 cm, and the thickness of silty deposits was 12–30 cm and their lower layer was black and had a smell of hydrogen sulfide. In addition to ammocoetes, macrobenthos organisms, such as small bivalve mollusks of the family Sphaeriidae, larvae of dragonflies (Odonata), and mayflies (Ephemeroptera), were caught. Sand mixed with silt and vegetative remains is the main component of the microbitope.
The analysis of algoflora in the microbiotope has shown that taxa that may be food objects for larval river lamprey differ in their ratio in sediments and water (Table 1). Bottom forms, species of the genera Navicula and Nitzschia, as well as Aulacoseira (67%), prevailed in the sample of sediments; Aulacoseira, Tabellaria, and Ulnaria (84%) were dominant in the water layer above the bottom sediments (0–1 cm above the surface); Aulacoseira (85%) prevailed in the main flow where Navicula and Nitzschia were absent.
Fullness of the digestive tract. A high degree of the intestine fullness, 2–4 points, was observed in most studied larvae (Table 1). The fullness of the gut sections was not always similar. The esophagus was full only in one larva, food remains were found in six specimens and the esophagus was empty in other specimens. The anterior section in 14 specimens contained a small amount of food (1–2 points), while its fullness was higher in other specimens (3–4 points). The middle and posterior sections of the intestine were usually filled densely and uniformly. The ratio of fullness of anterior, middle, and posterior sections may be estimated as 1 : 2 : 2.
Content of the digestive tract. The main mass of the content of all intestines was composed of sand and other particles of inorganic origin (usually >90%). Fine detritus (mainly of vegetative origin), pollen of higher plants (mostly of gymnosperms), algae, rotifers, small nematodes, crustaceans and their fragments were found in addition to inorganic particles.
Algae were mostly represented by diatoms (Bacillariophyta); in addition, blue-green algae (Cyanobacteria), charophyte green algae (Charophyta, an order of Desmidiales), and green algae (Chlorophyta), in particular, representatives of the classes of Protococcophyceae and Chlorococcales, were constantly present. The proportion of algae in the total mass of the bolus was small, within 0.03–1.50 (on average, 0.25 ± 0.07)% (Table 2). The basis was formed by diatoms, in particular species of the genera Aulacoseira, Navicula, Nitzschia, Tabellaria, Pinnularia, and Ulnaria, which occurred in 90–100% of the intestines and constituted >80% of the total mass of algae (Fig. 1).
The ratio of the cell number of dominant algae taxa slightly differed in the bolus of the examined larvae (Fig. 2a). The proportion of Aulacoseira sp. constituted 34–93 (64)% of the total number of cells and was higher than 80% in most specimens. The species of the genus Navicula formed 1–38 (17)%, while they exceeded 20% in only eight larvae; Nitzschia constituted 1–28 (8)% and usually did not exceed 10%. The mass fraction of cells of these taxa slightly differs (Fig. 2b): Aulacoseira, 0.1–59 (26)%, Navicula, 0.3–22 (11)%, and Nitzschia, 0.1–34 (7)%. Solitary cells of large algae may make up a considerable proportion in the total mass: Pinnularia sp., 0–99 (24)%, and Closterium sp., 0–26 (4)%. Species of the other genera of diatoms, Cymbella, Cyclotella, Fragilaria, Meridion, and several solitary recorded taxa, are constantly found in the intestines. Their total contribution did not usually exceed 1–2%, sometimes reaching 5% of the total mass. Green algae are represented by very small Protococcophyceae that are sometimes mass species in the content of the bolus; only Monoraphidium sp. of them was identified and counted; the species was recorded in 100% of larvae intestines but did not play a considerable role in the total number and mass of algae. Chlorococcales Scenedesmus sp. and Pediastrum sp. (Chlorophyta) and species of the genus Closterium (Charophyta) were frequent (25–40%). The mass of the representatives of the genus Closterium in some cases reached 20% of the total mass of algae despite the small number (usually no more than three cells per intestine).
Based on the results of the cluster analysis of the fraction of some algae taxa in the total mass two groups of lamprey larvae were distinguished in intestines (Fig. 3). The first group includes larvae in the bolus of which Aulacoseira sp. prevails (its proportion was >70% in the total abundance and ~50% in the mass); the second group includes larvae with the prevalence of bottom algae, mainly Navicula sp. and Nitzschia sp., in the diet. In the second group Aulacoseira sp. constituted ~50% of the abundance and 8% of the mass. The average sizes of larvae in these groups (71 ± 5.4 and 45 ± 6.0 mm) significantly differ according to the Mann–Whitney U test (p = 0.02).
In addition to algae, small invertebrates were present in larvae intestines. Rotifers, and in the first turn Keratella cochlearis, played the main role among them and occurred in 74% of intestines; the number of specimens of Keratella cochlearis varied from several to 103 specimens. In addition, Kellicotia longispina (43%) and Colurella colurus (47%) were present in most larval lamprey. Solitary specimens of Keratella quadrata, Brachionus angularis, and Bdelloidae indet. were found. Their proportion was 0.01–0.81 (0.12 ± 0.04)% of the total mass of the bolus. Two groups are distinguished on the dendrogram of the larvae similarity in the number of rotifers (Fig. 4).
Nematodes (usually solitary specimens) were recorded in 55% of larvae. Their proportion in the total mass of the bolus was 0.7–17.9 (2.8 ± 0.96)%.
Plankton crustaceans, cladocerans Bosmina sp. and Chydorus sp., and Copepoda at juvenile stages are solitary; their proportion in the total mass did not exceed 0.03%. Solitary specimens of bottom crustaceans of the family Harpacticoidae were found in three larval lamprey; they constituted up to 0.7% of the total mass.
DISCUSSION
The studied microbiotope is a typical habitat of larval lamprey of all ages. Low current velocity, shading by a steep bank, and higher vegetation from the effect of sunlight, organic debris, and thick silty sediments rich in organic compounds create conditions for larvae that are probably close to optimal ones. According to Nazarov et al. (2016) the number of larval lamprey in such a biotope may reach 100 ind/m2. Physical parameters of the biotope make it possible for not only typical benthos organisms but also other groups, including periphyton and plankton algae, rotifers, and crustaceans, which may be food objects of larval lamprey, to remain or constantly inhabit in it.
Larval lampreys are filter feeders in respect to the type of feeding. Inorganic particles and food enter the oral cavity with a water flow. Solid particles entangle into mucus and then enter the esophagus. The analysis of the bolus of ammocoetes from the Chernaya River has demonstrated that its content is typical for lamprey larvae and does not differ from the data obtained in other regions (Schroll, 1959; Manion, 1967; Hardisty and Potter, 1971; Moore and Potter, 1976; Sutton and Bowen, 1994; Makeeva et al., 2011; Nazarov, 2012; Loshakova and Knizhin, 2015). The digestive tract basically contains sand, fine detritus with vegetative remains, algae, and small invertebrates.
The experiments (Moore and Potter, 1976) have demonstrated that, despite a high content of detritus and, respectively, bacteria, algae are a more valuable food component. Ammocoetes that fed only on detritus grew slower than ammocoetes feeding on algae. The researchers consider that the amount of bacteria necessary for normal growth and development is absent in the environment; therefore, their role in the diet of lamprey larvae is negligible.
Many researchers indicate that ammocoetes feed namely on bottom algae, representatives of the genera Navicula, Nitzschia, Ulnaria (former name Synedra), Gomphonema, Cymbella, Fragilaria, Pinnularia, and Closterium. These taxa were found in intestines of examined ammocoetes.
Some researchers (Creaser and Hann, 1929; Wigley, 1959) suggested that, despite the mode of life of larval lamprey (dwelling in silty-sandy sediments of the stream), organisms from the flow play an important role in their diet, since the number of food objects in the intestine from the water column was higher than from sediments. In the opinion of other researchers (Hardisty and Potter, 1971), larval lampreys collect dead particles from the sediment surface that sank from the flow more often. During the experiment with larvae of Ichthyomyzon fossor, the prevalence of food resources of seston over detritus was demonstrated using the analysis of amino acids and an organic component (Yap and Bowen, 2003). The species of the genus Aulacoseira (former name Melosira) were not indicated as a food object of ammocoetes before. According to our data, these algae play a critical role in their diet (64 ± 4.4% of the total number and 26 ± 4.4% of the total mass). The genus Aulacoseira is a widespread form of phytoplankton (Round et al., 1990) in the Neva River in particular and other water bodies in northwestern Russia (Nikulina, 2008; Belova et al., 2011). It is indicated as not an abundant species when describing the communities of bottom algae (Gubelit, 2008). The concentration of Aulacoseira in samples of sediments was five times lower than in the water column (Table 1). A lower proportion of Aulacoseira in the total mass compared to abundance is due to the small size of its cells. Larger bottom algae with lower abundance yielded higher mass values. The mass of several cells of large Pinnularia sp. constituted more than 50% of the total mass of algae (on average 24 ± 4.7%) in some cases. Despite the fact that Ulnaria sp. is a form of periphyton, it is characterized by higher concentrations in the near bottom water layer.
In the literature, only Desmidiales, in particular Closterium sp., are indicated of green algae in the bolus. We recorded not less than two species of the genus. Despite the fact that the number of cells of algae of this genus does not usually exceed several cells, their proportion in terms of mass may be considerably higher, especially in small larvae (size classes I–II) due to large sizes. Low abundance of Closterium sp. in the bolus is probably due to the fact that the development of algae of this group in the middle of May did not reach its maximum or these algae were not typical for this biotope. This suggestion is confirmed by the data on considerable seasonal changes in the contribution of algae to the diet of ammocoetes (Schroll, 1957, 1959; Moore and Potter, 1976; Nazarov, 2012). In addition to Closterium sp., coenobia of Scenedesmus sp. and Pediastrum sp. were regularly found in intestines of ammocoetes, which is not reported in the literature sources. They are plankton-periphyton microorganisms unlike a typically bottom form Closterium sp. (Anisimova and Gololobova, 2006; Barinova et al., 2006). In general, the taxonomic composition of algae in the bolus content of ammocoetes is typical for water bodies of Karelia and other regions of northwestern Russia (Komulainen, 2003; Komulainen et al., 2006; Gubelit, 2008; Balashova et al., 2016).
The proportion of algae in the total mass of the bolus of the examined larvae varied within 0.03–1.47 (0.05)% that was lower than values reported in the literature by 0.14–1.5% on average, sometimes to 5% (Moore and Mallatt, 1980). This may be explained by a different composition of food. Thus, the publication by Moore and Mallatt (1980) reports a considerable prevalence of large algae of the genus Navicula; our data indicate that cell sizes of mass forms were much smaller.
We did not find any relationship between the proportion of algae in the bolus and the size of larvae. This does not confirm the idea of the dependence of selectivity of food objects on the larvae size. On the one hand, such dependence may be the result of different migration activity of larvae: after the end of dispersion at the morning twilight, larvae sank to the bottom and burrow into sediments not so deep as the larvae that did not disperse during the previous night (or this season). On the other hand, this may be due to isolation of habitats of larvae and algae in sediments: algae never penetrate deeply into the sediments. These suggestions require further studies. A significant dependence of the abundance of dominant algal taxa in the bolus on the body length of larvae is not found. The highest fraction of algae was found in intestines of larvae of size class II (Table 2).
The role of aquatic fauna in the diet of ammocoetes is known only in general terms. Nematodes and, occasionally, other bottom invertebrates are usually detected among food objects (Sterba, 1953; Mihail, 1962; Alvarez del Villar, 1966; Nazarov, 2012). Rotifers are reported in some works (Sterba, 1953; Alvarez del Villar, 1966) but without indication of the taxonomic composition, quantitative parameters, and ecological belonging. In our samples, Keratella cochlearis, Kellicotia longispina, and Colurella colurus were found in most ammocoetes. The first two species are typical representatives of zooplankton in most water bodies; they are a part of the complex of mass species of zooplankton in water bodies of Karelia Gerd, 1946; Grigoriev, 1965; Andronikova, 1996). A large number of these organisms was found in water samples taken above the sediments and in the flow. Colurella colurus is distributed in the near bottom layer and overgrowths of vegetation; it is usually found in small overgrown water bodies (Kutikova, 1970; Koste, 1978). The species is absent in our samples taken from the flow but is found in the near bottom water layer. Rotifers were not found in the samples of sediments. As the total number of rotifers in the bolus reached several tens of individuals, we cannot consider their entry into the intestine to be occasional. Rotifers were present in larvae of all sizes in all intestinal sections (Table 2). Their proportion in the content of the bolus is especially high in larvae of size classes II–III; especially in the group of larvae in the bolus of which Aulacoseira sp. was dominant, 0.24 ± 0.07 (3–119) vs. 0.015 ± 0.007 (0–4) rotifers/specimen in a Naviculaceae-Nitzschia group. This once more confirms the presence of differences in feeding of lamprey larvae.
Thus, not only detritus and bottom microorganisms play an important role in the diet of ammocoetes. A constant presence of typically plankton rotifers and algae of the genus Aulacoseira most likely indicates the consumption of organisms from the water column and near bottom layer, which confirms the previous suggestions (Creaser and Hann, 1929; Wigley, 1959; Yap and Bowen, 2003). We cannot consider the presence of plankton organisms to be occasional, first of all, because of their presence in large amounts in all intestinal sections in half of the examined larvae. The suggestion that they are dead organisms that sank to the bottom is unlikely, because green chloroplasts are visible in algae and well-preserved internal organs in rotifers. The presence of bottom and plankton forms of algae and invertebrates may be associated with behavioral habits of larvae: they feed when burrowing into sediments part of the time, but they periodically poke their heads out of sediments and water with plankton organisms gets into the intestine.
We did not find any significant pattern of changes in the food composition depending on the larvae size. Our data make it possible to suggest with some probability that the ratio of algae taxa in the bolus changes with an increase in the length: the proportion of Aulacoseira sp. increases and the proportion of Navicula sp., on the contrary, decreases. This may be due to different migration activity of larval lamprey (larvae of younger age disperse more actively) and different depths of their habitation in sediments.
ACKNOWLEDGMENTS
We are grateful to A.S. Demchuk (Zoological Institute, Russian Academy of Sciences) for useful advice and valuable comments; R.M. Gogorev (Komarov Botanical Institute, Russian Academy of Sciences) for the help in identifying algoflora and advice; A.A. Ulanova (Department of Earth Sciences, Uppsala University, Uppsala, Sweden), V.N. Mikheev (Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences), and D.L. Lajus (Saint Petersburg State University) for the discussion of the manuscript.
FUNDING
This study was supported by the Russian Science Foundation, project no. 14-14-01171.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
Alimov, A.F., Vvedenie v produktsionnuyu gidrobiologiyu (Introduction into Production Hydrobiology), Leningrad: Gidrometeoizdat, 1989.
Alvarez del Villar, J., Ictiologia michoacana, IV. Contribucion al conocimiento Biologico y systematico de las Lampreas de Jacona, Mich. Mexico, An. Esc. Nac. Cienc. Biol. (Mexico), 1966, vol. 13, pp. 107–144.
Andronnikova, I.N., Strikturno-funktsional’naya organizatsiya zooplanktona ozernykh ekosistem (Structure and Functions of Zooplankton of Lake Ecosystems), St. Petersburg: Nauka, 1996.
Anisimova, O.V. and Gololobova, M.A., Kratkii opredelitel’ rodov vodoroslei (Brief Guide for Identification of Algae), Moscow: Mosk. Gos. Univ., 2006.
Balashova, N.B., Kiselev, G.A., Stepanova, V.A., and Tobias, A.V., Diatoms of benthic algae of the south coast of Gulf of Finland (Lebyazhii Nature Reserve), Vestn. S.-Peterb. Univ., Ser. 3: Biol., 2016, no. 16, pp. 9–25.
Barinova, S.S., Medvedeva, L.A., and Anisimova, O.V., Bioraznoobrazie vodoroslei-indikatorov okruzhayushchei sredy (Biological Diversity of Algae-Environmental Indicators), Tel-Aviv: Pilies Studio, 2006.
Belova, M.A., Bol’shakova, V.A., Zaitseva, I.I., and Nefedova, E.D., Long-term monitoring of phytoplankton of the Neva River (1955–2010) as biological indicator of water quality of water supply source for St. Petersburg and Leningrad oblast, Materialy II Mezhdunarodnoi konferentsii “Bioindikatsiya v monitoringe presnovodnykh ekosistem” (Proc. II Int. Conf. “Bioindication in Monitoring of Freshwater Ecosystems”), St. Petersburg, 2011, pp. 79–84.
Bowen, S.H., Sutton, T.M., Yap, M.R., et al., Feeding Ecology and Habitat Use by Lampreys in Great Lakes Tributaries: Final Report of the Michigan Technological University Task Area Team, Houghton: Michigan Technol. Univ., 1998.
Creaser, C.W. and Hann, C.S., The food of larval lampreys, Pap. Mich. Acad. Sci., Arts Lett., 1929, vol. 10, pp. 433–437.
Evans, T.M. and Bauer, J.E., Identification of the nutritional resources of larval sea lamprey in two Great Lakes tributaries using stable isotopes, J. Great Lakes Res., 2016, vol. 42, no. 1, pp. 99–107. https://doi.org/10.1016/j.jglr.2015.11.010
Gerd, S.V., Planktonic complex of large lakes of Karelia and summer migrations of the vendace, Uch. Zap. Karel. Univ., 1946, vol. 1, pp. 305–344.
Grigor’ev, S.V., Lakes and rivers of Karelia and their features, in Fauna ozer Karelii (Fauna of Karelian Lakes), Moscow: Nauka, 1965, pp. 21–41.
Gubelit, Yu.I., Phytoperiphyton of the Neva River estuary, in Ekosistema estuariya r. Nevy: biologicheskoe raznoobrazie i ekologicheskie problemy (Ecosystem of the Neva River Estuary: Biological Diversity and Ecological Problems), Alimov, A.F. and Golubkov, S.M., Eds., Moscow: KMK, 2008, pp. 96–105.
Hardisty, M.W. and Potter, I.C., The behavior, ecology and growth of larval lampreys, in The Biology of Lampreys, Hardisty, M.W. and Potter, I.C., Eds., London: Academic, 1971, vol. 1, pp. 85–125.
Hollet, A.K., A feasibility study to determine the immediate source of carbon filtered by Petromyzon marinus ammocoetes from the Root River, Sault St. Marie through the use of stable isotope analysis, PhD Thesis, Waterloo, ON: Univ. of Waterloo, 1995.
Instruktsiya po sboru i obrabotke materiala dlya issledovaniya pitaniya ryb v estestvennykh usloviyakh (Instruction for Collection and Processing of the Materials for Analysis of Feeding of Fishes in Nature), Moscow: VNIRO, 1971, part 1.
Jolley, J.C., Silver, G.S., Whitesel, T.A., and Telles, L., Captive Rearing of Pacific Lamprey, Vancouver, WA: U.S. Fish Wildl. Service, 2012.
Komulainen, S.F., Metodicheskie rekomendatsii po izucheniyu fitoperifitona v malykh rekakh (Methodological Recommendations for Analysis of Phytoperiphyton in Small Rivers), Petrozavodsk: Karel. Nauchn. Tsentr, Ross. Akad. Nauk, 2003.
Komulainen, S.F., Chekryzheva, T.A., and Vislyanskaya, A.G., Al’goflora ozer i rek Karelii. Taksonomicheskii sostav i ekologiya (Algological Flora of Lakes and Rivers of Karelia: Taxonomic composition and Ecology), Petrozavodsk: Karel. Nauchn. Tsentr, Ross. Akad. Nauk, 2006.
Kutikova, L.A., Kolovratki fauny SSSR (Rotifers of the Fauna of USSR), Leningrad: Nauka, 1970.
Lebedev, N.V., Behavior of European anchovy in the stream and its migration, Rybn. Khoz. (Moscow), 1936, no. 9, pp. 27–32.
Loshakova, Yu.V. and Knizhin, I.B., Morphological characteristics and ecological peculiarities of nonparasitic lampreys of the genus Lethenteron (Petromyzontidae) from the Angara River basin, J. Ichthyol., 2015, vol. 55, no. 2, pp. 162–171. https://doi.org/10.1134/S0032945215010105
Makeeva, A.P., Pavlov, D.S., and Pavlov, D.A., Atlas molodi presnovodnykh ryb Rossii (Atlas of Juveniles of Freshwater Fishes of Russia), Moscow: KMK, 2011.
Mallatt, J., Laboratory growth of larval lampreys (Lampetra (Entosphenus) tridentata Richardson) at different food concentrations and animal densities, J. Fish. Biol., 1983, vol. 22, pp. 293–301.
Manion, P.J., Diatoms as food of larval lampreys in a small tributary of Northern Lake Michigan, Trans. Am. Fish. Soc., 1967, vol. 96, pp. 224–226.
Metodicheskie rekomendatsii po sboru i obrabotke materialov pri gidrobiologicheskikh issledovaniyakh na presnovodnykh vodoemakh. Zadachi i metody izucheniya ispol’zovaniya kormovoi bazy ryboi (Methodological Recommendations for Collection and Processing of the Materials in Hydrobiological Studies in Freshwater Reservoirs: Objectives and Study Methods of Food Reserves by Fishes), Leningrad: Zool. Inst., Akad. Nauk SSSR, 1984.
Mihail, N., Zur Biologie und Ökologie von Eudontomyzon danfordi, Zool. Anz., 1962, vol. 168, pp. 139–143.
Moore, J.W. and Mallatt, J.M., Feeding of larval lamprey, Can. J. Fish. Aquat. Sci., 1980, vol. 37, pp. 1658–1664.
Moore, J.W. and Potter, I.C., A laboratory study on the feeding of larvae of the brook lamprey Lampetra planeri (Bloch), J. Anim. Ecol., 1976, vol. 45, no. 1, pp. 81–90.
Nazarov, A.V., Morphobiological characteristics of Siberian brook lamprey in the central stream of the Yenisei River, Materialy VIII Vserosiskoi nauchno-tekhnicheskoi konferentsii “Molodezh’ i nauka” (Proc. VIII All-Russ. Sci.-Tech. Conf. “Youth and Science”), Krasnoyarsk: Sib. Fed. Univ., 2012. http://conf.sfu-kras.ru/sites/mn2012/section31.html.
Nazarov, D., Kucheryavyy, A., and Pavlov, D., Distribution and habitat types of the lamprey larvae in rivers across Eurasia, in Jawless Fishes of the World, Orlov, A.M. and Beamish, R., Eds., Cambridge: Cambridge Scholars, 2016, vol. 1, pp. 280–298.
Nikulina, V.N., Phytoplankton of the Neva River estuary, in Ekosistema estuariya r. Nevy: biologicheskoe raznoobrazie i ekologicheskie problemy (Ecosystem of the Neva River Estuary: Biological Diversity and Ecological Problems), Alimov, A.F. and Golubkov, S.M., Eds., Moscow: KMK, 2008, pp. 77–96.
Pavlov, D.S., Nazarov, D.Yu., Zvezdin, A.O., and Kucheryavyi, A.V., Downstream migration of early larvae of the European river lamprey Lampetra fluviatilis, Dokl. Biol. Sci., 2014, vol. 459, no. 1, pp. 344–347.
Pavlov, D.S., Zvezdin, A.O., Kostin, V.V., Tsimbalov, I.A., and Kucheryavyy, A.V., Temporal characteristics of downstream migration of smolts of the European river lamprey Lampetra fluviatilis in the Chernaya River, Biol. Bull., 2017, vol. 44, no. 3, pp. 290–295.
Radchenko, I.G., Kapkov, V.I., and Fedorov, V.D., Prakticheskoe rukovodstvo po sboru i ananlizu prob morskogo fitoplanktona (Practical Manual on Collection and Analysis of Samples of Marine Phytoplankton), Moscow: Mordvintsev, 2010.
Round, F.E., Crawford, R.M., and Mann, D.G., The Diatoms. Biology and Morphology of the Genera, Cambridge: Cambridge Univ. Press, 1990.
Schroll, F., Zur Ernahrungsbiologie der Ammocoeteslarven der Cyclostomen, Zool. Anz., 1957, vol. 159, pp. 75–78.
Schroll, F., Zur Ernahrungsbiologie der steirischen Ammocoeten Lampetra planeri (Bloch) und Eudontomyzon danfordi (Regan), Int. Rev. Hydrobiol., 1959, vol. 44, pp. 395–429. https://doi.org/10.1002/iroh.19590440121
Spletnitskaya, N.A., Gogorev, R.M., and Ivanov, M.V., Specific feeding of the White Sea culturing blue mussel (Mytilus edulis L.) by phytoplankton, Vestn. S.-Peterb. Univ., Ser. 3: Biol., 2008, no. 4, pp. 39–46.
Sterba, G., Die Physiologie und Histogenese der Schilddriise und des Thymus beim Bachneunauge (Lampetra planeri Bloch) als Grundlage phylogenetischer Studien über die Evolution der innsekretorischen Kiemendarmderivate, Wiss. Z.-Friedrich-Schiller-Univ. Jena: Naturwiss. Reihe, 1953, vol. 2, pp. 239–298.
Sutton, T.M. and Bowen, S.H., Significance of organic detritus in the diet of larval lampreys in the Great Lakes Basin, Can. J. Fish. Aquat. Sci., 1994, vol. 51, pp. 2380–2387.
Tsalolikhin, S.Ya., Weight determination of freshwater nematodes, in Evolyutsiya, sistematika, morfologiya i ekologiya svobodnozhivushchikh nematod (Evolution, Systematics, Morphology, and Ecology of Free-Living Nematodes), Platonova, T.A. and Tsalolikhin, S.Ya., Eds., Leningrad: Zool. Inst., Akad. Nauk SSSR. 1981, pp. 80–85.
Voigt, M., Rotatoria, Die Rädertiere Mitteleuropas. Überordnung Monogononta. Ein Bestimmungswerk, Berlin: Gebruder Borntraeger, 1978, vol. 1.
Wigley, R.L., Life history of the sea lamprey of Cayugaf Lake, New York, Fish. Bull., 1959, vol. 59, pp. 561–617.
Yap, M.R. and Bowen, S.H., Feeding by northern brook lamprey (Ichthyomyzon fossor) on sestonic biofilm fragments: habitat selection results in ingestion of a higher quality diet, J. Great Lakes Res., 2003, vol. 29, pp. 15–25.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by N. Ruban
Rights and permissions
About this article
Cite this article
Polyakova, N.V., Kucheryavyy, A.V., Pavlov, D.S. et al. Feeding Habits of Larval European River Lamprey Lampetra fluviatilis from the Chernaya River (Baltic Sea Basin). J. Ichthyol. 59, 216–224 (2019). https://doi.org/10.1134/S0032945219020164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945219020164





 ), Aulacoseira, (
), Aulacoseira, ( ), Navicula, (
), Navicula, ( ), Synedra, (◻), Nitzschia, (
), Synedra, (◻), Nitzschia, ( ), Tabellaria, (
), Tabellaria, ( ), Pinnularia, (
), Pinnularia, ( ), Closterium, (◼), others.
), Closterium, (◼), others.
