Abstract
Landmark-based morphometrics are examined to evaluate the population status of Schizopyge niger (Heckel, 1838) from Dal Lake and Jhelum River. A total of 180 individuals are collected from the two locations, and a truss network is constructed by interconnecting 12 landmarks to yield 30 distance variables that are extracted from digital images of specimens using tpsDig2 and PAST software. Transformed truss measurements are subjected to Principal Component Analysis (PCA), where the first component explains 78.43% of total variance, while the second and third components explain 4.62 and 2.91% of total variance respectively. The high component loadings are from the characters which mostly contribute to anterior half of body depth, middle portion of body depth, and head region. The bivariate plot of PC1 and PC2 clearly indicates the separation of the two populations. The cross validation of this analysis reveals that the percentage of correctly classified fishes is 100% both in Dal Lake as well as Jhelum River.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The snow trouts (schizothoracines), belonging to the family Cyprinidae, are believed to have migrated into lakes and streams of Kashmir from Central Asian watersheds bordered by inner and southern slopes of Hindukush, Karakoram, and inner ends of north western Himalayas and Suleiman Ranges (Sehgal, 1999). A total of 68 species of schizothoracines are recorded worldwide (He and Chen, 2006). In India, snow trouts are distributed in the cold waters of Jammu and Kashmir (Sunder and Bhagat, 1979), Assam and Eastern Himalayas through Bhutan and Sikkim at an altitude of 1180–3000 m above mean sea level (Chandra et al., 2012). Schizopyge niger (Heckel, 1838) locally called as “Ael Gad” or “Alghad” inhabit the cold streams and rivers in the inland waters of Kashmir besides Afghanistan and Pakistan. Originally the fish, which was put under genus Schizothorax by Heckel (1938), was later described under Schizopyge by Heckel (1943) while reorganising classification of schizothoracids. The present study considered the basionym Schizopyge in the light of recent work on fishes of Kashmir Valley by Kullander et al. (1999).
Morphometric variation between stocks can provide a basis for stock structure and may be applicable for studying short-term environmentally induced variation geared towards successful fisheries management. For fisheries science and management, the basic requirement is to identify discrete unit stocks (Cushing, 1968). Poor understanding of the fish and fishery management can lead to dramatic changes in the biological attributes and productivity of a species (Altukhov, 1981; Ricker, 1981; Smith et al., 1991).
The Truss Network Analysis entails the whole fish body in a uniform network which could possibly help in extracting morphometric differences within and between species (Turan, 2000). It is more useful in comparison to traditional morphometrics method; it has better data collection, and effective strategy for the descriptions of shape (Dwivedi and Dubey, 2013). Several authors (Cheng et al., 2010; Hossain et al., 2010; Nahar et al., 2013) emphasized on the validity of the truss network of morphometric characters which enforces systematic coverage of the form and also exhaustively and redundantly archives the landmark configuration. This work was conducted to determine the morphological differentiation between riverine and lacustrine populations of S. niger in Kashmir using landmark based truss network analysis.
MATERIALS AND METHODS
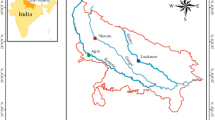
Study area. Jhelum River, the major water body of Kashmir, is the tributary of Indus basin and flows in western Himalayan region of India (Fig. 1). It originates in the Kashmir Valley, about 54 km east of Anantnag, and is 816 km long with a catchment area of 39200 km2. The river has ten major tributaries including Neelum, Kishan Ganga (the largest tributary 260 km long with a catchment area of 3968 km2), Kunhar, Poonch, and Kanshi. Taking its origin from a perennial spring known as Verinag, the river meanders north-westward from the northern slope of the Pir Panjal Range through the valley to Wular Lake, which controls its flow. It is the second largest fisheries resource of the valley after Wular Lake and harbours, a wide variety of fish fauna including both indigenous and exotic species (Sodhi et al., 2013).
Dal Lake is known as the “Jewel of Kashmir”, and is situated in the north-east of Srinagar at mean latitude of 34°06′ N and longitude of 74°52′ N at an altitude of 1584 m (Fig. 1). The lake covers an area of about 11.4 km2 with maximum depth of 5.4 m and functions as the central part of large interconnected aquatic ecosystems in Kashmir Valley (Dar and Romshoo, 2008).
Sample collection and digitization. A total of 180 individuals of S. niger in the length range of 188 to 338 mm (61 to 287 g) and 199 to 325 mm (106.5 to 305 g) were collected at random from commercial catches of the two selected sites namely Jhelum River (34°08′88.26″ N, 74°79′05.71″ E; n = 90) and Dal lake (34°14′60.83″ N, 74°84′39.61″ E; n = 90), respectively from December 2016 to May 2017. The samples were placed individually in plastic bags and transported to the laboratory in ice boxes. Since male and female fish could not be differentiated morphologically, sexing of the fish that were sampled was not carried out. The samples were first cleaned in running water, drained, and placed on a flat platform. Digital images of individual fish (on the left side of fish) were taken immediately after collecting them from the landing center. To capture the images, a digital camera (Sony Cybershot 10X) was used which provided the complete image of body shape and allowed the repeat of measurements when necessary (Cadrin and Friedland, 1999). For calibrating the coordinates of digital images, graph paper was used as a background.
Measurement of truss distances. A truss network was constructed by interconnecting 12 landmarks to form a total of 30 inter-landmark distances (Fig. 2). Three softwares, namely tps Dig2 V2.1, Paleontological Statistics (PAST) (Hammer et al., 2001), and tpsUtil V1.38 were used for the extraction of truss distances from the digital images of specimens. All the images were first converted from JPEG (*.jpeg) to TPS (*.tps) format by using a utility program called tpsUtil V1.38 and specifying the ruler. The data encrypted TPS format image files from tps Dig V2.1 were used as input source in the PAST, and the data on distances between the coordinates were extracted.
Analysis of data. As most shape measurements are in some way related to size, any heterogeneity in the size across the samples would result in heterogeneity in the shape, but without providing information on differences in body proportions among populations (Reist, 1985). There are significant correlations between body length and truss measurements. Therefore, transformation of absolute measurements to size-independent shape variables was the first step of the analysis, and it was done by modifying a formula originally given by Ihssen et al. (1981) and Hurlbut and Clay (1998). Size-dependent variation for truss variables was removed using the formula: Dtrans= D × (SLmean/SL)b. Where, Dtrans is the transformed truss measurement, D is the original truss measurement, SL is the standard length of fish, SLmean is the overall mean standard length (25.2 mm), b is the within group slope of the geometric mean regression calculated with log-transformed variables, D and SL. In the present study principal component analysis (PCA) was employed to discriminate the two populations. Principal Component Analysis helps in morphometric data reduction (Mir et al., 2013), in decreasing the redundancy among the variables (Samaee et al., 2006), and in extracting the independent variables for population differentiation (Samaee et al., 2009).
RESULTS
The correlation coefficient between truss measurements and standard length was highly significant before the transformation for size correction. After transformation of the truss measurements, the correlation coefficients were considerably reduced. It indicates that the effect of body length has been successfully removed with the allometric transformation. In order to determine the morphometric measurements that most effectively differentiate the species, the contributions of variables to principal components (PC) were examined. Principal component analysis of 30 morphometric measurements extracted three Principal components with eigenvalues >1 explaining 85.96% of total variation where the first principal component (PC1) accounted for 78.43% of total variance, while the second principal component (PC2) and third principal component (PC3) accounted for 4.62 and 2.91% of total variation respectively. In this analysis, the characteristics with eigenvalues exceeding 1 were included and others discarded.
The truss distances with meaningful loading on PC1 were 3-4, 3-9, 3-10, 4-9, 4-10 and 9-10. All these six distances characterize the measurements of the middle portion of the fish body depth. Four out of the 30 variables studied in the present work showed significant loadings on PC2, the distances were 2-3, 2-10, 3-11 and 10-11, which characterise the measurements of anterior half of the fish body depth, while variables 1-2, 1-11, 1-12, 2-11, 2-12, and 11-12 contributed to PC3 belong to the head region (Table 1). With respect to the location, the bivariate plot of PC1 and PC2 clearly indicates the separation of the two populations (Fig. 3). The cross validation of this analysis revealed that 100% individuals correctly classified to Dal Lake and Jhelum River, respectively.
DISCUSSION
The observed morphological variation among riverine and lacustrine S. niger samples revealed the existence of two morphologically differentiated stocks. The high component loadings were from the characters which mostly contributed to anterior half of body depth, middle portion of body depth, and head region. Morphometrics of the head and body depth have been regarded as the most important characters for discrimination of fish populations, for example angler fish Lophius vormernus (Leslie and Grant, 1990), Pacific herring Clupea pallasi (Schweigert, 1990), and orange roughy Hoplostethus atlanticus (Haddon and Willis, 1995). The pattern of high inter-sample morphological variation, as observed in the present study, may indicate reproductive isolation between local populations that would confirm the genetic basis of observed morphometric differentiation among samples (Turan, 2004). Geographical isolation can result in the development of different morphological features between fish populations because of the interactive effects of environment, selection, and genetics on individual ontogenies to produce morphometric differences within a species (Poulet et al., 2005). Fish are very sensitive to changes in environmental conditions and quickly adapt themselves by changing necessary morphometrics (Allendorf and Phelps, 1988; Wimberger, 1992). There are notable differences in the environmental factors such as temperature, food availability, and water velocities between Jhelum River and Dal lake (Yousuf and Shah, 1988; Qadri and Yousuf, 1988; Pandit, 1996; Lone et al., 2013; Akhtar et al., 2015). The variation in the middle portion and anterior half of the body can be explained by the effect of water velocity in the two studied aquatic ecosystems. The differences in the water velocities between the lake and the river is a significant factor influencing morphological divergence in the inhabitant fishes. Dal Lake is a lentic water body while Jhelum River is a lotic ecosystem and thus, has more turbulent water conditions (Khan and Sabah, 2013). Many fishes show distinct morphological differences between lotic and lentic habitats (Robinson and Wilson, 1994; Brinsmead and Fox, 2002). Fishes with streamlined morphologies are better able to overcome hydrodynamic drag in high flow environments (Blake, 1983). Many authors have reported that body, head and fins in fish are highly affected by water velocity (Pakkasmaa and Piironen, 2001; Solem et al., 2006; Nofrita et al., 2015). Parvej et al. (2014) reported morphological differences in riverine and lacustrine populations of Eutropiichthys vacha in Bangladesh. However, significant morphological differences do not necessarily demonstrate restrictions of gene flow among populations, though they do suggest that fish in each group may not mix extensively (Turan, 2004). Diet has also been shown to cause variation in morphology not only in fish but also in most organisms (Meyer, 1988). The difference in the head region may reflect differential habitat use, and variations in the head region are considered to be the result of differences in feeding regimes (Gatz, 1979). This could probably be due to difference in food types within the river systems. As morphology is especially dependent on environmental condition during early life-history stages (Ryman et al., 1984; Cheverud, 1988), morphological differentiation may indicate that the majority of fish spend their entire lives in different regions (Turan, 2004).
This study provides basic information about morphological differentiation of S. niger populations using morphometric parameters. The stock in Dal lake was confirmed to be separated from that of Jhelum River and, therefore, the populations may be considered as two self-contained stocks. There is a need for separate management strategies to sustain the stocks for future use. Development of proper guidelines for implementation of appropriate mesh size in both locations may help to sustain this resource for future use. This study also recommends adoption of modern approaches such as molecular genetics and biochemical methods in future to validate and substantiate the findings from this work.
ACKNOWLEDGMENTS
We are grateful to Dr. Sajna Ali, ICAR-CIFRI, West Bengal, India and Mr Mudasir Maqsood, Ph.D. Scholar (Animal Biotechnology), F.V.Sc. and A.H., SKUAST-Kashmir, Shuhama, Ganderbal, Jammu and Kashmir, India, for their help in the study.
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
REFERENCES
Akhtar, M., Muni, P., and Qayoom, I., Limnological features of Dal Lake of Kashmir Valley with special reference to the anthropogenic pressure along its water shed, Ecol. Environ. Conserv., 2015, vol. 21, no. 4, pp. 497–803.
Allendorf, F.W. and Phelps, S.R., Loss of genetic variation in hatchery stock of cutthroat trout, Trans. Am. Fish. Soc., 1988, vol. 109, pp. 537–543.
Altukhov, Yu.P., The stock concept from the viewpoint of population genetics, Can. J. Fish. Aquat. Sci., 1981, vol. 31, pp. 1523–1538.
Blake, R.W., Fish Locomotion, Cambridge: Cambridge Univ. Press, 1983.
Brinsmead, J. and Fox, M.G., Morphological variation between lake- and stream-dwelling rock bass and pumpkin populations, J. Fish. Biol., 2002, vol. 61, pp. 1619–1638.
Cadrin, S.X. and Friedland. K.D., The utility of image processing techniques for morphometric analysis and stock identification, Fish. Res., 1999, vol. 43, nos. 1–3, pp. 129–139.
Chandra, S., Barat, A., Singh M., Singh B.K., and Matura, R., DNA bar-coding of Indian cold water fishes of genus Schizopyge (family: Cyprinidae) from Western Himalaya, World J. Fish Mar. Sci., 2012, vol. 4, no. 4, pp. 430–435.
Cheng, H.L., Lui, T.Y., and Cheng, Y.H., Morphometric variation between the swordtip (Photololigo edulis) and mitre (P. chinensis) squids in the waters off Taiwan, J. Mar. Sci. Technol., 2010, vol. 18, no. 3, pp. 405–412.
Cheverud, J.M., A comparison of genetic and phenotypic correlations, Evolution, 1988, vol. 42, pp. 958–968.
Cushing, D.H., Fisheries Biology: A Study in Population Dynamics, Madison: Wisconsin Univ. Press, 1968.
Dar, A.A. and Romshoo, S.A., Assessing the hydrological characteristics of Dal Lake catchment using GIS, Proc. The 12th World Lake Conf., Sengupta, M. and Dalwani, R., Eds., Jaipur, 2008, pp. 659–667.
Dwivedi, A.K. and Dubey, V.K., Advancements in morphometric differentiation: a review on stock identification among fish populations, Rev. Fish Biol. Fish., 2013, vol. 23, no. 4, pp. 23–39.
Gatz, A.J., Community organization in fishes as indicated by morphological features, Ecology, 1979, vol. 60, no. 4, pp. 711–718.
Haddon, M. and Willis T.J., Morphometric and meristic comparison of orange roughy (Hoplosethus atlanticus: Trachichthyidae) from the Puysegur Bank and Lord Howe Rise, New Zealand and its implications for stock structure, Mar. Biol., 1995, vol. 123, no. 1, pp. 19–27.
Hammer, O., Harper, D.A.T., and Ryan, P.D., PAST: Paleontological statistics software package for education and data analysis, Palaeontol. Electron., 2001, vol. 4, no. 1, pp. 1–9.
He, D. and Chen, Y., Biogeography and molecular phylogeny of the genus Schizopyge (Teleostei: Cyprinidae) in China inferred from cytochrome b sequences, J. Biogeogr., 2006, vol. 33, vol. 8, pp. 1448–1460.
Heckel, J.J., Fishes aus Cashmir, Gesammelt und Herausgegeben von Carl Freiherrn von Hügel, Beschrieben von J. J. Heckel, Vienna, 1838, pp. 1–112.
Heckel, J.J., Ichthyologie, in Reisen in Europa, Asien and Afrika, mit Besonderer Rucksicht auf die Naturwissenschaflichen Verhaltnisse der betreffenden Lande, Unternommen in del Jahren 1835 bis 1841, Russegger, J., Ed., Stuttgart, 1843, vol. 1, ch. 2, pp. 991–1099.
Hossain, M.A.R., Nahiduzzaman, M., Saha, D., Khanam, M.U.H., and Alam, M.S., Landmarks-based morphometric and meristic variations of the endangered carp, Kalibaus, Labeo calbasu, from stocks of two isolated rivers, the Jamuna and Halda and a hatchery, Zool. Stud., 2010, vol. 49, pp. 556–563.
Hurlbut, T. and Clay, D., Morphometric and meristic differences between shallow and deep-water populations of white hake (Urophycis tenuis) in the southern Gulf of Lawrence, Can. J. Fish. Aquat. Sci., 1998, vol. 55, no. 10, pp. 2274–2282.
Ihssen, P.E., Booke, H.E., Casselman, J.M., McGlade, J.M., Payne, M.R., and Utter, F.M., Stock identification: materials and methods, Can. J. Fish. Aquat. Sci., 1981, vol. 38, no. 12, pp. 1838–1855.
Leslie, C.C. and Grant, W.S., Lack of congruence between genetic and morphological stock structure of the Southern African anglerfish Lophius vomerinus, South Afr. J. Mar. Sci., 1990, vol. 9, pp. 379–398.
Lone, S.A., Faried, A., Lori, S.M., and Zubar, S.M., Physicochemical characterization of lotic systems of Kashmir: a case study of River Jhelum, Int. J. Sci. Nat., 2013, vol. 4, no. 4, pp. 579–582.
Khan M.A. and Sabah, Length-weight and length-length relationships for five fish species from Kashmir Valley, J. Appl. Ichthyol., 2013, vol. 29, no. 1, pp. 283–284.
Kullander, S.O., Fang, F., Delling, B., and Ahlander, E., The Fishes of the Kashmir Valley, in River Jhelum, Kashmir Valley: Impacts on the Aquatic Environment, Nyman, L., Ed., Goteborg: Swedmar, 1999, pp. 99–167.
Meyer, A., Influence of age and size on the response to novel prey by fry of the cichlid fish Cichlasoma managuense (Pisces: Cichlidae), Ethology, 1988, vol. 78, no. 3, pp. 199–208.
Mir, F.A., Mir, J.I., and Chandra, S., Phenotypic variation in the snowtrout Schizopyge richardsonii (Gray, 1832) (Actinopterygii: Cypriniformes: Cyprinidae) from the Indian Himalayas, Contrib. Zool., 2013, vol. 82, no. 3, pp. 115–122.
Nahar, K., Hossain, M., Begum, A., Sultana, N., and Khan, M.G.Q., Genetic structure of endangered bata (Labeo bata, Hamilton) inferred from landmark-based morphometric and meristic measurements and allozyme markers, Int. Res. J. Pharm. Appl. Sci., 2013, vol. 3, no. 5, pp. 145–160.
Nofrita, D., Syandri, H., and Tjong, D.H., Morphological differentiation between bilih fish (Cyprinidae: Mystacoleucus padangensis, Bleeker) in Singkarak Lake and Anai River, West Sumatra, Indonesia, J. Entomol. Zool. Stud., 2015, vol. 3, no. 5, pp. 171–175.
Pakkasmaa, S. and Piironen, J., Morphological differentiations among local trout (Salmo trutta) populations, Biol. J. Linn. Soc., 2001, vol. 72, pp. 231–239.
Pandit, A.K., Lakes in the Kashmir Himalaya, in Ecology, Environment and Energy, Khan, A.H. and Pandit, A.K., Eds., Srinagar: Univ. of Kashmir, 1996, pp. 1–40.
Parvej, M.R., Islam, M.R., Minar, M.H., Hossain, M.B., and Tushar, M.R., Landmark-based morphometric and meristic variations of critically endangered catfish, Eutropiichthys vacha from three different populations in Bangladesh, World J. Fish Mar. Sci., 2014, vol. 6, no. 4, pp. 378–385.
Poulet, N., Reyjol, Y., Collier, H., and Lek, S., Does fish scale morphology allow the identification of population of Leuciscus burdigalensis in River Viaur, Aquat. Sci., 2005, vol. 67, no. 1, pp. 122–127.
Qadri, M.Y. and Yousuf, A.R., A comparative study of the limnology of three typical water bodies of Kashmir, in Recent Advances in Fish Ecology, Limnology and Eco-Conservation, Nath, S., Ed., New Delhi: Creative, 1988, pp. 79–91.
Reist, J.D., An empirical evaluation of several univariate methods that adjust for size variation in morphometric data, Can. J. Zool., 1985, vol. 63, no. 6, pp. 1429–1439.
Ricker, W.E., Changes in the average size and age of pacific salmon, Can. J. Fish. Aquat. Sci., 1981, vol. 38, pp. 1636–1656.
Robinson, B.W. and Wilson, D.S., Character release and displacement in fishes: a neglected literature, Am. Nat., 1994, vol. 144, no. 4, pp. 596–627.
Ryman, N., Lagercrantz, U., Anderson, L., Chakraborty, R., and Rosenberg, R., Lack of correspondence between genetic and morphological variability patterns in Atlantic herring (Clupea harengus), Heredity, 1984, vol. 53, pp. 687–704.
Samaee, S.-M., Mojazi-Amiri, B., and Hosseini-Mazinani, S.M., Comparison of Capoeta capoeta gracilis (Cyprinidae, Teleostei) populations in the south Caspian Sea river basin, using morphometric ratios and genetic markers, Folia Zool., 2006, vol. 55, pp. 323–335.
Samaee, S.-M., Patzner, R.A., and Mansour, N., Morphological differentiation within the population of Siah Mahi, Capoeta capoeta gracilis, (Cyprinidae, Teleostei) in a river of the south Caspian Sea basin: a pilot study, J. Appl. Ichthyol., 2009, vol. 25, pp. 583–590.
Schweigert, J.F., Comparison of morphometric and meristic data against truss networks for describing Pacific herring stocks, in Fish–Marking Techniques, Parker, N.C., et al., Eds., American Fisheries Society Symposium Series vol. 7, Bethesda: Am. Fish. Soc., 1990, pp. 47–62.
Sehgal, K.L., Coldwater Fish and Fisheries in the Indian Himalayas: Culture, FAO Fisheries Technical Paper no. 385, Rome: Food Agric. Org., 1999, pp. 89–102.
Smith, P.J., Francis, R., and McVeagh, M., Loss of genetic diversity due to fishing pressure, Fish. Res., 1991, vol. 10, pp. 309–316.
Sodhi, A.S., Saroch, J.D., and Verma, J., Fisheries resources of Kashmir: a case study of River Jhelum, J. Chem. Biol. Phys. Sci., 2013, vol. 3, no. 2, pp. 1194–1200.
Solem, O. Berg, O.K., and Kjoonest, A.J., Inter- and intra- population morphological differences between wild and farmed Atlantic salmon juveniles, J. Fish. Biol., 2006, vol. 69, pp. 1466–1481.
Sunder S. and Bhagat M.J., A note on the food of Schizopyge plagiostomus (McClelland) in the Chenab drainage of Jammu Province during 1973–1974, J. Inland Fish. Soc. India, 1979, vol. 11, no. 1, pp. 117–118.
Turan, C., Otolith shape and meristic analysis of herring (Clupea harengus) in the northeast Atlantic, Arch. Fish. Mar. Res., 2000, vol. 48, no. 3, pp. 283–295.
Turan, C., Stock identification of Mediterranean horse mackerel (Trachurus mediterraneus) using morphometric and meristic characters, ICES J. Mar. Sci., 2004, vol. 61, no. 5, pp. 774–781.
Wimberger, P.H., Plasticity of fish body shape, the effects of diet, development, family and age in two species of Geophagus (Pisces: Cichlidae), Biol. J. Linn. Soc., 1992, vol. 45, pp. 197–218.
Yousuf, A.R. and Shah, G.M., Comparative limnology of some freshwater habitat of Kashmir, Geobios, 1988, vol. 7, pp. 58–61.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Gul, S., Shah, T.H., Bhat, B.A. et al. Morphological Differentiation Between Riverine and Lacustrine Populations of Snow Trout Schizopyge niger (Cyprinidae) from Kashmir using Truss Morphometry. J. Ichthyol. 59, 160–166 (2019). https://doi.org/10.1134/S0032945219020085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945219020085