Abstract
Using the Cr3C2–NiAl cathode prepared by self-propagating high-temperature synthesis, Cr‒Ni–Al–C–N coatings are deposited by pulsed cathode arc evaporation in argon, nitrogen, and ethylene atmospheres. The coating structures are investigated by scanning electron microscopy, X-ray diffraction analysis, glow-discharge optical emission spectroscopy, and Raman spectroscopy. Mechanical and tribological properties are investigated by nanoindentation and pin-on-disk tribological testing. Anticorrosion properties are evaluated by voltammetry in the medium of a 1-N H2SO4 solution. The nonreactive coating deposited in an inert Ar atmosphere contains the Cr3C2 fcc phase with a size of crystallites below 20 nm. The deposition in the C2H4 and N2 reaction media leads to amorphization of the coatings. The samples deposited in argon and nitrogen exhibit high hardness values of 24–25 GPa. The deposition of coatings in C2H4 gives rise to a 40% decrease in the hardness. However, the carbon-containing sample has a relatively low coefficient of friction at a level of 0.28, as well as better wear and corrosion resistances due to the positive effect of the diamond-like carbon phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
High-current technologies, such as high-power pulsed magnetron sputtering [1], electrospark alloying (ESA), and pulsed cathodic arc evaporation (PCAE) [2] with use of ceramic or composite electrode materials prepared by self-propagating high-temperature synthesis (SHS), are widely used at present to apply protective coatings for various purposes (wear-, heat- and corrosion-resistant coatings). Severe degradation of ceramics due to thermal shock can be prevented using a pulsed power supply [2–4]. The deposition of a thin surface layer makes it possible to modify a wide range of substrates and provide them with increased hardness, wear resistance, and corrosion resistance characteristic of bulk ceramic, cermet, and composite materials [5–7]. The main advantages of these technologies include high productivity and high adhesive strength of the obtaining coatings [8]. The high adhesion strength of the coating to the substrate is caused either by metallurgical reactions at the interface in the case of ESA [9], or by bombardment of the substrate with high-energy ions and the formation of extended pseudodiffusion layers between the substrate and the growing coating in the case of PCAE [10]. The frequency and duration of the pulse, the mean and maximum power values, the discharge energy, and other electrical characteristics are key parameters of the deposition process for controlling the properties of coatings [11, 12]. The structure and properties can be additionally controlled using various gaseous media during the deposition of coatings [13, 14].
Chromium carbide is among the most promising materials for applying protective coatings. Coatings based on the Cr3C2–NiCr system [15–17] are widely known; new compositions with improved characteristics, such as Cr3C2–ZrO2 [18], Cr3C2–NiCr–CeO2 [19], Cr3C2–NiMo [20], and Cr3C2–NiCrCoMo [21], are also being developed. Previously, we investigated the Cr3C2–NiAl coatings deposited by the ESA method in a flow of argon or air under standard conditions [22]. The deposition of coatings by magnetron sputtering and electrospark alloying in vacuum was studied [23]. It was shown that coatings synthesized under optimal conditions exhibit high wear resistance and a low coefficient of friction at the level of 0.18. Samples deposited by magnetron sputtering of Cr3C2–NiAl cathodes in argon are characterized by high denseness and uniformity [22]. It is known that the introduction of additional components by deposition in a reaction atmosphere can improve the technological characteristics of coatings. It should be noted that the Cr–Ni–Al–C–N coatings synthesized by the PCAE method in a reaction medium have not been studied previously.
This study is devoted to the synthesis of coatings by the PCAE method with evaporation of the Cr3C2–NiAl cathode in various gas media, such as argon, nitrogen, and ethylene, and to the investigation of the effect of the working medium on the properties of the deposited coatings.
MATERIALS AND TECHNIQUES
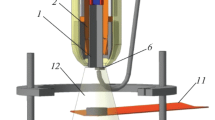
In this study, a Cr3C2–NiAl cathode obtained by self-propagating high-temperature synthesis was used for evaporation. Compared to the powder metallurgy methods, SHS is characterized by better technoeconomic parameters, such as high productivity, relatively low cost achieved through using cheaper raw materials, and the purity of the obtained products. Disks made from 40Ch steel (analog of 5140 steel) (chemical composition: 97.0 wt % Fe, 0.36–0.44 wt % C, 0.17–0.37 wt % Si, 0.5–0.8 wt % Mn, 0.8–1.1 wt % Cr, and 0.3 wt % Cu) with a diameter of 30 mm and a thickness of 5 mm were used as substrates. The coatings were synthesized on a setup based on an UVN-2M pumping system, in the chamber of which a PCAE unit equipped with a cathode made of the deposited material, ring-shaped and spiral-shaped metallic anodes, and an ignition electrode was installed (Fig. 1).
The cathode was placed in a special holder and isolated from electrical breakdowns by means of a Teflon tube and a quartz flask. Ignition was carried out using a metal wire. The anode voltage was supplied to a water-cooled copper coil and a disc made of copper. The substrate was fixed in a ring holder in the space between the spiral and the disk at a distance of 20 mm from the cathode. The energy of the arc discharge was accumulated in a battery of capacitors with a capacity of 2000 μF. To release this energy in an arc discharge, a high-voltage initiating pulse supplied to the cathode was used. The ignition voltage and frequency were 15 kV and 10 Hz, respectively. The voltage applied to the anode was close to 110 V, while the current reached 1 kA. The pressure of the working gases, i.e., Ar (99.9995%), N2 (99.999%), and C2H4 (99.95%), was 0.4 Pa, and the residual pressure was 4 × 10–3 Pa. The substrates were ultrasonically cleaned in isopropyl alcohol. Next, an anode voltage was applied to the substrate after placing it in a vacuum chamber for the first 2–3 min to heat it by electron bombardment. Then, a pseudodiffusion layer was formed for 5 min with a smooth decrease in the negative bias voltage applied to the substrate from –2 to 0 kV. Before depositing coatings in reaction media, the sublayer was deposited by evaporation of the cathode in argon for 1–2 min.
For structure analysis, a Hitachi S-3400N scanning electron microscope with a NORAN System 7 energy dispersive analysis system was used. The distribution profiles of elements were obtained using a Profiler-2 HORIBA-JY optical emission spectrometer in accordance with the procedure described in [24]. X‑ray diffraction analysis was performed on a Bruker D8 ADVANCE diffractometer with use of a CuKα radiation source. The size of crystallites was calculated using the Debye–Scherrer formula. Raman spectra were recorded on an NTEGRA NT-MDT instrument with use of a red laser (wavelength 633 nm). Mechanical characteristics, such as hardness H, elasticity modulus E, and elastic recovery W, were investigated on a Nanohardness Tester (CSM Instruments) under a load of 4 mN. The parameters were calculated using the Oliver–Pharr method. The hardness values were calculated as the ratio of the maximum load to the projection area of the recovered indentation; the elastic modulus was determined on the basis of the indentation projection area, the contact stiffness calculated from the slope of the upper third of the unloading curve, Poisson’s ratio, and the parameters of the indenter. Tribological tests were carried out on a Tribometer automated friction machine (CSM Instruments) by the pin-on-disk method with an Al2O3 counterbody at a linear velocity of 10 cm/s and under a load of 1 N. Fractographic analyses of wear grooves with subsequent calculation of normalized wear were performed on a Veeco WYKO NT1100 optical profilometer. For experimental determination of corrosion resistance, a three-electrode cell with a Voltalab PST050 potentiostat was used. The tests were carried out a 1-N solution H2SO4 at a temperature of 25°C.
RESULTS AND DISCUSSION
The coating compositions determined by glow discharge optical emission spectroscopy (GDOES) can be written as follows: Cr47.0Ni5.2Al1.3C46.5 (coating 1), Cr37.8Ni7.8Al1.4C10.3N42.7 (coating 2), and Cr20.8Ni6.0Al3.0C70.2 (coating 3). Pronounced inclusions of a condensed droplet phase were observed on the surface of all coatings, which appeared as a result of melting and evaporation of the cathode material (Fig. 2a). This kind of a surface defect is a feature of cathode arc coatings [25]. For all coatings, the concentration of predominantly spherical droplets was within 13–15% of the coating area.
It should be noted that the coating thicknesses determined from the GDOES profiles were 1.0, 0.6, and 2.3 μm for coatings 1, 2, and 3, respectively.
According to SEM images of transverse fractures, all coatings showed a similar dense homogeneous structure. A droplet phase is noticeable on the coating surfaces; no defects were observed inside the coatings. In reactive coatings, a sublayer deposited in an Ar medium is clearly observed (Fig. 2b).
According to the XRD data, the X-ray diffraction patterns of all coatings contain the peaks of the Fe substrate (JCPDS 06-0696) and the Ni(Al,Cr) solid solution (JCPDS 16-17228) (Fig. 2c). For coatings synthesized in Ar and N2, the peaks attributed to reflections from the (201), (211), and (121) planes of the Cr3C2 fcc phase (JCPDS 89-2723) were found. The size of Cr3C2 crystallites in samples 1 and 2 did not exceed 25 nm. The precipitation in an N2 reaction medium additionally led to the formation of the Cr2N phase (JCPDS 35-0803). Peaks from the Cr7C3 phase (JCPDS 071-3789) were revealed in the case of sputtering in a C2H4 medium, the size of crystallites in which was 30–50 nm. In the range 2Θ = 35°–50°, a broadened peak was observed, which is probably associated with the formation of amorphous carbon.
The Raman spectra of the Cr–Ni–Al–C–N coatings deposited in Ar, N2, and C2H4 atmospheres are shown in Fig. 2d. No characteristic peaks were found for the coatings deposited in Ar and N2. At the same time, the Raman spectra of the coating obtained in a C2H4 medium exhibit peaks at 1330 and 1520 cm–1, the shapes and positions of which are similar to those of the diamond-like carbon (DLC) phase [26].
The study of mechanical characteristics showed that the coating deposited in argon has a hardness of H = 24 ± 5 GPa, a maximum elastic modulus of E = 305 ± 37 GPa, and an elastic recovery of W = 59% (Table 1).
In the case of coating deposition in an N2 medium, the hardness value remained practically unchanged and was 25 ± 5 GPa; however, a decrease in the elastic modulus by 8% was observed. In this case, sample 2 had the maximum elastic recovery at W = 70%. Similar results, i.e., an insignificant increase in the hardness and a decrease in the elastic modulus with an increase in the nitrogen concentration, were obtained for the Al–Cr–B–N cathode arc coatings in [27]. Precipitation in an ethylene medium led to decreases in the hardness by about 40% and in the elastic modulus by about 35–40%. At the same time, the elastic recovery of coating 3 was 66%, which is 12% higher than the values obtained for the nonreactive coating. The decrease in the hardness in the case of depositing coatings in a carbon-containing medium can be associated with the formation of a graphite phase. A similar phenomenon was observed in [28, 29]. The H/E and H3/E2 characteristics were also determined using the nanoindentation results (Table 2), which can serve as parameters of the wear resistance and the extent of coating destruction [30].
Graphs of the dependence of the friction coefficient (f) on the distance and the mean f values of coatings 1–3 are shown in Fig. 3 and Table 2. The presence of a running-in stage at a running distance of 0–14 m, after which f smoothly increases to 0.70–0.76 and remains constant until the end of the test, is a characteristic feature of coating 1. The coefficient of friction of the nitrogen-containing coating after 40 m of the test increased to about 1.0 and remained constant for the rest of the test distance.
A jump of f to 0.44 was observed at a range of 0–25 m in the case of coating 3, which is associated with the running-in stage of the coating. The carbon-containing coating showed a low and stable coefficient of friction at about 0.28. A decrease in the friction coefficient in the case of deposition in ethylene can be associated with the formation of graphite on the coating surface, which is confirmed by the Raman spectroscopy data (Fig. 2d). As was shown in [31, 32], graphite contributes to a decrease in the friction coefficient in the region of tribological contact on a worn surface due to the lubricating effect.
According to the 2D and 3D profiles, the depth of the wear track (hw) of coating 1 obtained in Ar was 5 μm at a thickness of 1 μm, which indicates complete wear of sample 1. For sample 2, hw = 1.5 μm, which exceeds the thickness of the coating and indicates complete wear. The coating obtained in C2H4 with a thickness of 2.3 µm was characterized by hw = 0.3 µm. It can be concluded that sample 3 is virtually not worn out.
It should be noted that the coating wear rates (Vw) determined from the 3D profiles of the friction tracks (Fig. 4) decreased about 13 and 3 times upon the transition from sputtering in Ar and N2 to sputtering in a C2H4 medium, respectively (Table 2).
The polarization curves for coatings 1–3 are shown in Fig. 5.
The corrosion potentials (φ) and corrosion current density (icor) calculated using the Tafel equation are summarized in Table 2. Close values of the corrosion potentials of all tested samples indicate that the current densities of the coatings are mainly determined by dissolution of the substrate material (iron). For a nonreactive coating, the corrosion current density was 88 μA/cm2. Upon switching to reactive sputtering in an N2 medium for coating 2, the corrosion current density increased 2 times and reached 189 μA/cm2. The sample deposited in a C2H4 medium was characterized by a minimum corrosion current density of 35 μA/cm2. Thus, the coating synthesized in C2H4 showed the corrosion resistance 2.5 and 5.4 times higher than the corrosion resistances of the samples deposited in Ar and N2, respectively.
CONCLUSIONS
Using the method of pulsed cathode arc evaporation of the Cr3C2–NiAl electrode, coatings are synthesized in Ar, N2, and C2H4 atmospheres. The coating deposited in Ar contains the Cr3C2 and Ni(Al,Cr) phases. The transition to sputtering in an N2 reaction medium facilitates the formation of the additional Cr2N phase in the coating. The deposition in C2H4 leads to a change in the dominant phase from Cr3C2 to Cr7C3; as a result of the excess of C, a broadened peak that corresponds to amorphous carbon is observed in the X-ray diffraction pattern. The samples deposited in Ar and N2 show comparable hardness values of 24 and 25 GPa, respectively. In the case of depositing coatings in a carbon-containing environment, the coating hardness decreases by 40% as a result of the formation of a soft phase of graphite. However, the formation of free carbon leads to a decrease in the coefficient of friction and an increase in the wear resistance of the coating in comparison with the samples deposited in argon and nitrogen. The coating deposited in C2H4 is characterized by a low corrosion current at the level of 35 μA/cm2, which is 2.5 and 5.4 times less than the current density values obtained for the samples deposited in Ar and N2, respectively.
Thus, the coatings deposited in Ar and N2 exhibit the best mechanical properties. At the same time, the carbon-containing sample has a low coefficient of friction, and high values of the wear and corrosion resistances.
REFERENCES
Ph. V. Kiryukhantsev-Korneev, A. N. Sheveyko, S. A. Vorotilo, and E. A. Levashov, “Wear-resistant Ti–Al–Ni–C–N coatings produced by magnetron sputtering of SHS-targets in the DC and HIPIMS modes,” Ceram. Int. 46, 1775–1783 (2019).
Ph. Kiryukhantsev-Korneev, A. Sytchenko, A. Sheveyko, D. Moskovskikh, and S. Vorotylo, “Two-layer nanocomposite TiC-based coatings produced by a combination of pulsed cathodic arc evaporation and vacuum electro-spark alloying,” Materials 13, No. 547 (2020).
X. Zhao, Z. Chen, H. Wang, Z. Zhang, G. Shao, R. Zhang, B. Fan, H. Lu, H. Xu, and D. Chen, “The influence of additive and temperature on thermal shock resistance of ZrB2 based composites fabricated by Spark Plasma Sintering,” Mater. Chem. Phys. 240, No. 122061 (2020).
M. Antonov and I. Hussainova, “Thermophysical properties and thermal shock resistance of chromium carbide based cermets,” Proc. Estonian Acad. Sci. Eng. 12, 358–367 (2006).
E. Almandoz, De Ara J. Fernandez, J. De Bujanda Martinez, J. Palacio Fernandez, R. Jose Rodriguez, Z. Zhang, H. Dong, Y. Qin, and G. Garcia Fuentes, “CrAlON CAE-PVD coatings for oxidation and wear protection of TZM alloys in FAST sintering applications,” Mater. Chem. Phys. 208, 189–197 (2018).
V. S. Goncharov, E. V. Vasil’ev, and M. V. Goncharov, “Effect of technological parameters of applying yttrium-containing coatings on their structure and properties,” Phys. Metals. Metallogr. 115, 169–174 (2014).
R. Krause-Rehberg, A. D. Pogrebnyak, V. N. Borisyuk, M. V. Kaverin, A. G. Ponomarev, M. A. Bilokur, K. Oyoshi, Y. Takeda, V. M. Beresnev, and O.V. Sobol’, “Analysis of local regions near interfaces in nanostructured multicomponent (Ti–Zr–Hf–V–Nb)N coatings produced by the cathodic-arc-vapor-deposition from an arc of an evaporating cathode,” Phys. Met. Metallogr. 114, 672–680 (2013).
Ph. V. Kiryukhantsev-Korneev and K. A. Kuptsov, “Impact wear-resistance of Ti–Cr–B–N coatings produced by pulsed CAE of ceramic target,” J. Phys.: Conf. Ser. 1238, No. 012003 (2019).
H. Shafyei, M. Salehi, and A. Bahrami, “Fabrication, microstructural characterization and mechanical properties evaluation of Ti/TiB/TiB2 composite coatings deposited on Ti6Al4V alloy by electro-spark deposition method,” Ceram. Int. 46, 15276–15284 (2020).
P. E. Hovsepian and A. P. Ehiasarian, “Six strategies to produce application tailored nanoscale multilayer structured PVD coatings by conventional and High Power Impulse Magnetron Sputtering (HIPIMS),” Thin Solid Films 688, No. 137409 (2019).
MH. Mei, J. C. Ding, X. Xiao, Q. Luo, R. Wang, Q. Zhang, W. Gong, and Q. Wang, “Influence of pulse frequency on microstructure and mechanical properties of Al–Ti–V–Cu–N coatings deposited by HIPIMS,” Surf. Coat. Technol. 405, No. 126514 (2021).
F. Ferreira, R. Serra, J. C. Oliveira, and A. Cavaleiro, “Effect of peak target power on the properties of Cr thin films sputtered by HiPIMS in deep oscillation magnetron sputtering (DOMS) mode,” Surf. Coat. Technol. 258, 249–256 (2014).
J. F. Kiryukhantsev-Korneev and K. A. Pierson, “Hard Cr–Al–Si–B–(N) coatings deposited by reactive and non-reactive magnetron sputtering of CrAlSiB target,” Appl. Surf. Sci. 314, 104–111 (2014).
SD. V. Shtansky, N. A. Gloushankova, A. N. Sheveiko, P. V. Kiryukhantsev-Korneev, I. A. Bashkova, B. N. Mavrin, S. G. Ignatov, S. Y. Filippovich, and C. Rojas, “Si-doped multifunctional bioactive nanostructured films,” Surf. Coat. Technol. 205, 728–739 (2010).
M. Shi, Z. Xue, H. Liang, Z. Yan, X. Liu, and S. Zhang, “High velocity oxygen fuel sprayed Cr3C2–NiCr coatings against Na2SO4 hot corrosion at different temperatures,” Ceram. Int. 46, 23629–23635 (2020).
Y. Zhang, K. Chong, Q. Liu, Y. Bai, Z. Zhang, D. Wu, and Y. Zou, “High-temperature tribological behavior of thermally-treated supersonic plasma sprayed Cr3C2-NiCr coatings,” Int. J. Refract. Met. Hard Mater. 95, No. 105456 (2021).
H. Lu, J. Shang, X. Jia, Y. Li, F. Li, J. Li, and Y. Nie, “Erosion and corrosion behavior of shrouded plasma sprayed Cr3C2–NiCr coating,” Surf. Coat. Technol. 388, No. 125534 (2020).
V. Suresh, A. Jegan, and S. L. Kumar, “Microstructure, mechanical and tribological characteristics of plasma and HVOF sprayed Cr3C2–PS.ZrO2 coatings,” Mater. Today: Proc. 33, 1137–1143 (2020).
S. Kumar, D. Mudgal, S. Singh, and S. Prakash, “Effect of CeO2 in Cr3C2–NiCr Coating on Superni 600 at High Temperature,” Procedia Mater. Sci. 6, 939–949 (2014).
W. Zhai, B. Pu, L. Sun, Y. Wang, H. Dong, Q. Gao, L. He, and Y. Gao, “Influence of molybdenum content and load on the tribological behaviors of in-situ Cr3C2–20 wt % Ni composites,” J. Alloys Compd. 826, 154180 (2020).
J.-Y. Du, Y.-L. Li, F.-Y. Li, X.-J. Ran, X.-Y. Zhang, and X.-X. Qi, “Research on the high temperature oxidation mechanism of Cr3C2–NiCrCoMo coating for surface remanufacturing,” J. Mater. Res. Technol. 10, 565–579 (2021).
Ph. V. Kiryukhantsev-Korneev, A. D. Sytchenko, V. A. Gorshkov, and E. A. Levashov, “Mass-transfer kinetics, structure, and tribological properties of coatings deposited on steel in Ar or N2 + O2 by electro-spark alloying using Cr3C2–NiAl electrodes,” IOP Conf. Ser.: Mater. Sci. Eng. 848, No. 012087 (2020).
Ph. V. Kiryukhantsev-Korneev, N. V. Shvyndina, A. D. Sytchenko, D. V. Shtansky, V. A. Gorshkov, and E. A. Levashov, “Healing effect in coatings deposited by hybrid technology of vacuum electro-spark alloying, pulsed cathodic arc evaporation, and magnetron sputtering using Cr3C2–NiAl electrodes,” J. Phys.: Conf. Ser. 1431, No. 012027 (2020).
F.V. Kiryukhantsev-Korneev, “Possibilities of glow discharge optical emission spectroscopy in the investigation of coatings,” Rus. J. Non-Ferrous Met. 55, 494–504 (2014).
P. J. Martin and A. Bendavid, “Review of the filtered vacuum arc process and materials deposition,” Thin Solid Films 394, 1–14 (2001).
J. Laumer, K. Schmidt, and S. K. O’Leary, “The relationship between the Raman spectral form and the location of the corresponding sample within the overall thin-film carbon genome,” Solid State Commun. 322, No. 114059 (2020).
B. Warcholinski, A. Gilewicz, P. Myslinski, E. Dobruchowska, D. Murzynski, P. Kochmanski, K. Rokosz, and S. Raaen, “Effect of nitrogen pressure and substrate bias voltage on the properties of Al–Cr–B–N coatings deposited using cathodic arc evaporation,” Tribol. Int. 154, No. 106744 (2021).
F. V. Kiryukhantsev-Korneev and A. V. Bondarev, “Structure and properties of antifriction Cu, Cu–C, and DLC coatings,” Phys. Met. Metallogr. 120, 702–708 (2019).
M. M. Khrushchev, E. A. Marchenko, I. S. Levin, V. M. Avdyukhina, M. D. Reilyanu, E. A. Obraztsova, and M. V. Atamanov, “Structure and tribological behavior of chromium-carbon coatings obtained by magnetron sputtering,” Phys. Met. Metallogr. 120, 204–209 (2019).
J. Musil, F. Kunc, H. Zeman, and H. Polakova, “Relationships between hardness, Young’s modulus and elastic recovery in hard nanocomposite coatings,” Surf. Coat. Technol. 154, 304–313 (2002).
Z. Guo, A. Zhang, J. Han, and J. Meng, “Microstructure, mechanical and tribological properties of CoCrFeNiMn high entropy alloy matrix composites with addition of Cr3C2,” Tribol. Int. 151, No. 106436 (2020).
M. H. Staia, T. Valente, C. Bartuli, D. B. Lewis, C. P. Constable, A. Roman, J. Lesage, D. Chicot, and G. Mesmacque, “Part II: tribological performance of Cr3C2–25% NiCr reactive plasma sprayed coatings deposited at different pressures,” Surf. Coat. Technol. 146–147, 563–570 (2001).
Funding
In part of electrochemical studies, this work was supported by the Russian Science Foundation (contract no. 20-79-10104).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by O. Kadkin
Rights and permissions
About this article
Cite this article
Kiryukhantsev-Korneev, P.V., Sytchenko, A.D., Sheveyko, A.N. et al. Effect of a Gas Medium on the Mechanical, Tribological, and Anticorrosion Properties of Cr–Ni–Al–C–N Coatings Deposited by the Pulsed Cathodic Arc Evaporation Method. Phys. Metals Metallogr. 122, 1241–1247 (2021). https://doi.org/10.1134/S0031918X21120048
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X21120048