Abstract
Capabilities of modern methods of computer simulation of the phase composition and structure of the low-alloyed steels with carbonitride hardening at the solidification, austenization, and hot deformation of an ingot are briefly reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
High-strength low-alloyed steels with additions of strong carbonitride-forming (V, Nb, and Ti) elements have been worked out since the middle of the last century. The field of application of such steels is constantly widening, requirements to their characteristics are steadily growing, and new publications on the studies of these materials are constantly appearing [1‒5]. Usually more than ten elements are involved in composition of a steel, and its treatment as a rule includes several stages, which makes it necessary to carry out a great number of experimental studies. Though it is not possible to do without any experimental studies at all, recently various methods of computer simulations have been more and more widely used in working out new steel grades and their heat treatments.
The goal of the present paper is a brief review of capabilities of computer simulation for the prediction of the phase composition and structure of low-alloyed steels with carbonitride hardening at solidification, austenization, and hot deformation, based on the authors’ original studies and available publications.
SIMULATION OF CARBONITRIDE FORMATION AT AN INGOT SOLIDIFICATION
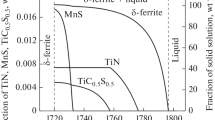
Titanium nitrides are practically not soluble in steels in solid state and their precipitation starts at solidification of an ingot. Regularities of formation of carbonitride phase composition in steels with Ti were studied in [6–8]. These studies showed that in the cast state two types of nitride particles are formed, coarse particles (the sizes of 1–10 μm), formed from a liquid phase before and in the process of crystallization, and dispersed ones (the size of up to 10 nm), precipitating from the solid solution under cooling. Besides, in steels, possessing an excess Ti compared to N, Ti sulphides are formed, and a part of Ti is retained in the solid solution. The effect of different type particles on the structure and mechanical properties of steels is not the same [7, 8]. Dispersed nitrides can effectively retard the growth of austenite grains under heating, whereas the formation of coarse nitrides and sulphides causes embrittlement.
To predict an effect of a steel composition on the amount of particles of different types, a step-by-step algorithm, allowing to simulate the process of crystallization in steels with titanium, was worked out in [9]. The main idea of the suggested algorithm is that the crystallization process is divided into a great number of steps, in each of which the amount of a solid phase is increased by δq. When δq → 0 this model approaches the real equilibrium crystallization process. It was suggested that at every step a local equilibrium is established between a liquid phase and a new portion of a solid phase. It was also suggested that concentrations of elements in a solid phase formed earlier remained unchanged, except that of carbon. In these calculations, the possibility of precipitation of such compounds, as TiN, TiS, and MnS from the crystallizing liquid phase was taken into account. This model allows simulation of solidification process of steels crystallizing with the formation of δ-ferrite (<0.1% C), steels with peritectic transformation (0.1–0.51% C), and steels crystallizing with austenite formation (0.51–2.06% C). Comparison of calculation results with experiment demonstrated their good agreement. Such calculations make it is possible to predict the amount of coarse nitrides and sulphides of titanium and sulphides of manganese, as well as concentrations of elements in a solid solution after the solidification. In its turn, if Ti and N concentrations in the solid solution are known, one can approximately predict the amount of dispersed titanium nitrides.
PREDICTION OF PHASE COMPOSITION AND STRUCTURE AT AUSTENIZATION
The majority of thermal and thermomechanical treatments of low-alloyed steels (normalization, hardening with tempering, controlled rolling) include an operation of austenization. That is why to choose an optimal composition and treatment schedule of a steel one must be able to predict its phase composition, the state of precipitate ensemble, and the grain sizes at heating into the austenite range.
Thermodynamic Calculations
The use of thermodynamic calculations considerably simplifies the problem of finding optimal compositions of steels and their treatment schedules. By now, information on several programming products for thermodynamic calculations has been published, including [10, 11]. Some of these packages are distributed on the commercial basis. However, firstly, they are quite expensive and, secondly, it is unknown whether the algorithms on which these packages are based, are enough reliable. In [12] we worked out an algorithm for calculations of phase equilibriums in multiphase multicomponent systems. This algorithm is based on the search of global minimum of Gibbs energy of a multicomponent alloy with some restrictions in the form of inequalities and equalities. For the search of local minimums in this algorithm a special procedure of choosing starting points is realized, ensuring combination of acceptable calculation productivity and high probability of the global minimum finding. The thermodynamic description of multicomponent systems was based on the CALPHAD method described in [13]. The Hillert and Staffonsson model [14], later generalized by Sundman and Ågren [15], was used for the description of thermodynamic properties of Fe-based solid solutions and interstitial phases of alternating compositions.
In [16–23], thermodynamic descriptions were constructed, and, using the algorithm from [12], the thermodynamic calculations were performed for a number of systems modelling low-alloyed steels. Particularly, in [22, 23] the thermodynamic description was made, taking into account the probability of the presence in a steel along with Fe, C and N of three strong carbonitride-forming elements (V, Nb, and Ti) and such alloying elements as Al, Cr, Mn, Ni, and Si. Thus, an opportunity appeared to carry out thermodynamic calculations for real commercial low-alloyed steels. Such calculations make it easier to choose optimal composition and treatment schedule of a steel. Particularly, in [24] thermodynamic calculations were used in choosing the controlled rolling schedules for a tubular billet on Mill-5000 of the Magnitogorsky metallurgical plant.
Kinetic Simulation
Thermodynamic calculations enable one to find an equilibrium phase composition of a steel and to estimate the effect of the temperature and composition on it. However, firstly, at heat treatment not always an equilibrium phase composition is reached, and, secondly, the influence of carbonitride precipitates on the structure is determined not only by the volume fraction of carbonitride phases. Of much greater importance is the average particle size, and, to be more exact, the particle size distribution. That is why there appeared a great number of publications on simulation of precipitate evolution and their effect on the structure [25‒28]. However, in the majority of these works such important factors as poly-dispersity of precipitate ensemble and possibility of overlapping of diffusion fields from different precipitates were not taken into account. The method suggested in [29] and based on the mean-field approximation,—is free from this drawback. This approximation assumes formulation of particle growth rate in every size category through interaction between a particle and its average surrounding. This means that interaction between a particle and matrix in field cells is considered, and it is suggested that concentrations of components on the boundaries of all field cells \(X_{i}^{L}\) are similar. Substance exchange between particles occurs through the field cell walls. Geometrical scheme of a model of precipitate evolution based on the mean-field approximation is shown in Fig. 1.
In [30–32] this method was generalized on a case of multicomponent alloys, in [33] the formation of new nucleation centers was taken into account, and in [34] diffusion in precipitates was taken into consideration, as well. In our recent studies this method was extended on multiphase systems [35–37].
Prediction of Austenite Grain Size
By now there exist various theoretical models predicting effect of the second phase particles on the matrix grain growth [38, 39]. These models use an approach based on the finding of a hypothetic critical grain size, when the moving force for the growth of such grains is exactly balanced with the pinning force exerted by the particles. In [40] we analyzed possibilities of the austenite grain size prediction using these models, based on the results of calculations of a precipitate ensemble state. Comparison of the results of these calculations with the available experimental data showed their satisfactory agreement. The best agreement of calculations and experiment is reached when Gladman model [41] is used for high-temperature range (1150–1250°C), corresponding to the temperatures of heating for rolling.
SIMULATION OF PRECIPITATE EVOLUTION AND AUSTENITE GRAIN SIZE UNDER HOT DEFORMATION
Great interest in working out models for prediction of the formation of the structure and properties of steels under hot plastic deformation is not weakening (see, e.g., [42–44]). The most complete model of the existing ones describing the microstructure evolution and taking into account the effect of precipitates on the recrystallization kinetics is given in [44]. The main drawback of this study is that an approach used for simulation of precipitate evolution is far from being perfect.
In our recent publications [45, 46] a model free from this drawback was worked out for austenite structure evolution under hot deformation and recrystallization. This model consists of three interconnected parts. The first one, based on an approach suggested in [47], describes the evolution of dislocation density both in the process and after deformation. The second part describes kinetics of changing of an average grain size of austenite. Here the consideration is based on an expression from [42], modified to take into account the effect of carbonitride precipitates. The third part describes kinetics of the formation and evolution of carbonitride precipitates using our approach of consideration of precipitate evolution in multiphase and multicomponent systems worked out in [35–37]. Comparison of the results of calculations and experiment demonstrates that the model suggested enables to predict satisfactorily both the state of a precipitate ensemble, and the austenite grain size under hot deformation and recrystallization.
CONCLUSIONS
The modern state of studies in computer simulation of phase composition and structure of low-alloyed steels with carbonitride hardening at crystallization, austenization, and hot deformation is briefly reviewed. It is demonstrated that methods and approaches worked out presently can adequately enough describe the processes occurring at crystallization, austenization, and hot deformation, and in most cases demonstrate a satisfactory agreement with an experiment. Thus, the computer simulation methods can be recommended in working out new steels and optimization of their treatment schedules.
REFERENCES
K. Wu, Z. Li, A. M. Guo, XL. He, L. Zang, F. Fang, and L. Cheng, “Microstructure evolution in low carbon Nb–Ti microalloyed steels,” ISIJ Int. 46, 161–165 (2006).
C. Y. Chen, C. C. Chen, and J. R. Yang, “Microstructure characterization of nanometer carbides heterogeneous precipitation in Ti–Nb,” Mater. Charact. 69, 69–79 (2014).
V. M. Schastlivtsev, T. I. Tabatchikova, I. L. Yakovleva, S. Yu. Del’gado Reina, S. A. Golosienko, U. A. Pazilova, and E. I. Khlusova, “Effect of thermomechanical treatment on the resistance of low-carbon low-alloy steel to brittle fracture,” Phys. Met. Metallogr. 116, 189–199 (2015).
V. M. Farber, V. A. Khotinov, S. V. Belikov, O. V. Selivanova, N. V. Lezhnin, A. N. Morozova, M. S. Karabonalov, and A. Yu. Zhilyakov, “Separations in steels subjected to controlled rolling, followed by accelerated cooling,” Phys. Met. Metallogr. 117, 407–421 (2016).
O. V. Sych, A. A. Kruglova, V. M. Schastlivtsev, T. I. Tabatchikova, and I. L. Yakovleva, “Effect of vanadium on the precipitation strengthening upon tempering of a high-strength pipe steel with different initial structure“ Phys. Met. Metallogr. 117, 1270–1280 (2016).
L. Meyer, H. E. Buhler, and F. Heisterkamp, “Metallkundliche Untersuchangen zur Wirkungsweise von Titan in unlegierten Baustahlen,” Arch. Eisenhuttehw. 43, 823–832 (1972).
M. I. Goldshteyn, L. P. Zhitova, and V. V. Popov, “Influence of titanium carbonitrides on the structure and properties of low-carbon steels,” Fiz. Met. Metalloved. 51, 1245–1252 (1981).
J.-Y. Li and W.-Y. Zhang, “Effect of TiN inclusion on fracture toughness in ultrahigh strength steel,” ISIJ Int. 29, 158–164 (1989).
V. V. Popov and A. O. Homenko, “Similation of phase formation in cast steels with titanium,” Izv. Akad. Nauk, Met. No. 4, 82–88 (1994).
J. O. Anderson, “Thermo-Calk & Dictra computational tools for materials science,” CALPHAD 26, 273–312 (2002).
C. W. Bale, P. Chartrand, and S. A. Degterov, “FactSage Thermochemical Software and Databases,” CALPHAD 26, 189.
V. V. Popov and I. I. Gorbachev, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: I. Description of thermodynamic properties. Computation procedure,” Phys. Met. Metallogr. 98, 344–354 (2004).
H. L. Lukas, S. G. Fries, and B. Sundman, Computational Thermodynamics: The CALPHAD Method (Cambridge University, Cambridge, 2007).
M. Hillert and L.-I. Staffonsson, “The regular solution model for stoichiometric phases and ionic melts,” Acta Chem. Scand. 42, 3618–3626 (1970).
B. Sundman and J. Ågren, “A regular solution model for phase with several components and sublattices suitable for computer applications,” J. Phys. Chem. Solids 42, 297–301 (1981).
V. V. Popov and I. I. Gorbachev, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: II. Solubility of carbides, nitrides, and carbonitrides in the Fe–V–C, Fe–V–N, and Fe–V–C–N systems,” Phys. Met. Metallogr. 99, 286–299 (2005).
I. I. Gorbachev and V. V. Popov, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: III. Solubility of carbides, nitrides, and carbonitrides in the Fe–Ti–C, Fe–Ti–N, and Fe–Ti–C–N systems,” Phys. Met. Metallogr. 108, 484–495 (2009).
I. I. Gorbachev and V. V. Popov, “Analysis of solubility of carbides, nitrides, and carbonitrides in steels using methods of computer thermodynamics: IV. Solubility of carbides, nitrides, and carbonitrides in the Fe–Nb–C, Fe–Nb–N, and Fe–Nb–C–N systems,” Phys. Met. Metallogr. 110, 52–61 (2010).
I. I. Gorbachev and V. V. Popov, “Thermodynamic simulation of the Fe–V–Nb–C–N system using the CALPHAD method,” Phys. Met. Metallogr. 111, 495–502 (2011).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic simulation of the formation of carbonitrides in steels with Nb and Ti,” Phys. Met. Metallogr. 113, 687–695 (2012).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic simulation of the formation of carbonitrides in steels with V and Ti,” Phys. Met. Metallogr. 113, 974–981 (2012).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Thermodynamic calculations of carbonitride formation in low-alloy low-carbon steels containing V, Nb, and Ti,” Phys. Met. Metallogr. 115, 69–76 (2014).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Calculations of the influence of alloying elements (Al, Cr, Mn, Ni, Si) on the solubility of carbonitrides in low-carbon low-alloy steels,” Phys. Met. Metallogr. 117, 1226–1236 (2016).
V. M. Salganik, A. V. Shmakov, and V. V. Popov, “Rational controlled rolling on a 5000 pipe-blank mill at reduced temperature,” Steel in Translation 39, 906–911 (2009).
J. Svoboda, F. D. Fischer, P. Fratzl, and E. Kozeschnik, “Modelling of kinetics in multicomponent multiphase systems with spherical precipitates I: Theory,” Mater. Sci. Eng. A 385, 166–174 (2004).
E. Kozeschnik, J. Svoboda, and F. D. Fischer, “Modified evolution equations for the precipitation kinetics of complex phases in multicomponent systems,” CALPHAD 28, 379–382 (2004).
P. B. S. Srinivas, V. B. Rajkumar, and K. C. Hari Kumar, “Numerical simulation of precipitate evolution in ferritic–martensitic power plant steels,” CALPHAD 36, 1–7 (2012).
S. Shahandech and M. Militzer, “Grain boundary curvature and grain growth kinetics with particle pinning,” Philos. Mag. A 93, 3231–3247 (2013).
V. V. Popov, “Numerical simulation of the evolution of a polydisperse ensemble of precipitates in a two-component alloy upon isothermal annealing,” Phys. Met. Metallogr. 87, 379–386 (1999).
V. V. Popov, “Simulation of dissolution and coarsening of MnS precipitates in Fe–Si,” Philos. Mag. A 82, 17–27 (2002).
V. V. Popov, “Simulation of the evolution of precipitates in dilute alloys,” Phys. Met. Metallogr. 93, 303–309 (2002).
V. V. Popov and I. I. Gorbachev, “Simulation of the evolution of precipitates in multicomponent alloys,” Phys. Met. Metallogr. 95, 417–426 (2003).
V. V. Popov, I. I. Gorbachev, and J. A. Alyabieva, “Simulation of VC precipitate evolution in steels with consideration for the formation of new nuclei,” Philos. Mag. 85, 2449–2467 (2005).
I. I. Gorbachev, V. V. Popov, and E. N. Akimova, “Computer simulation of the diffusion interaction between carbonitride precipitates and austenitic matrix with allowance for the possibility of variation of their composition,” Phys. Met. Metallogr. 102, 18–28 (2006).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Simulation of evolution of precipitates of two carbonitride phases in Nb and Ti containing steels during isothermal annealing,” Phys. Met. Metallogr. 114, 741–751 (2013).
I. I. Gorbachev, V. V. Popov, and A. Yu. Pasynkov, “Simulation of precipitate ensemble evolution in steels with V and Nb,” Phys. Met. Metallogr. 116, 356–366 (2015).
V. V. Popov, I. I. Gorbachev, and A. Y. Pasynkov, “Simulation of precipitates evolution in multiphase multicomponent systems with consideration of nucleation,” Philos. Mag. 96, 3632–3653 (2016).
P. A. Manohar, M. Ferry, and T. Chandara, “Five decades of the Zener equation,” ISIJ Int. 38, 913–924 (1998).
G. S. Thompson, “Kinetic model of particle-inhibited grain growth,” A Dissertation for the Degree of Doctor of Philosophy (Lehigh University, 2001).
I. I. Gorbachev, A. Yu. Pasynkov, and V. V. Popov, “Prediction of the austenite-grain size of microalloyed steels based on the simulation of the evolution of carbonitride precipitates,” Phys. Met. Metallogr. 116, 1127–1134 (2015).
T. Gladman, “On the theory of the effect of precipitate particles on grain growth in metals,” Proc. R. Soc. (London), Ser. A 294, 298–309 (1966).
R. Sandrom and R. Lagneborg, “A model for hot working occurring by recrystallization,” Acta Metall., 23, 387–398 (1975).
C. Roucoules and M. Pietrzyk, and P. D. Hodgson, “Analysis of work hardening and recrystallization during the hot working of steel using a statistically based internal variable model,” Mater. Sci. Eng., A 339, 1–9 (2003).
A. Timoshenkov, P. Warczok, M Albu, J. Klarner, E. Kozeschnik, R. Bureau, and C. Sommitsch, “Modelling the dynamic recrystallization in C–Mn micro-alloyed steel during thermo-mechanical treatment using cellular automata,” Comput. Mater. Sci, 24, 85-94 (2014).
I. I. Gorbachev, A. Yu. Pasynkov, and V. V. Popov, “Simulation of the effect of hot deformation on the austenite grain size of low-alloy steels with carbonitride hardening,” Phys. Met. Metallogr. 119, 551–557 (2018).
I. I. Gorbachev, A. Yu. Pasynkov, and V. V. Popov, “Simulation of the evolution of carbonitride particles of complex composition during hot deformation of low-alloy steel,” Phys. Met. Metallogr. 119, 770–779 (2018).
M. Pietrzyk, “Through-process modelling of microstructure evolution in hot forming of steels,” J. Mater. Process. Technol. 125–126, 53–62 (2002).
ACKNOWLEDGMENTS
The work has been done within the state assignment on the topic Spin АААА-А18-118020290104-2 and in the framework of the project no. 18-10-2-37 of the Ural Branch of the Russian Academy of Sciences Program Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Popov, V.V., Gorbachev, I.I. Computer Simulation for the Prediction of Phase Composition and Structure of Low-Alloyed Steels with Carbonitride Hardening. Phys. Metals Metallogr. 119, 1333–1337 (2018). https://doi.org/10.1134/S0031918X18130252
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X18130252