Abstract
The results of the phylogenetic analysis and analysis of the physiological characteristics of metabolic activity of strains isolated from frozen sedimentary rocks collected in the Antarctic and the Severnaya Zemlya archipelago are reported in this article. A comparable abundance of cultured cells and close morphological diversity of colony morphotypes were revealed in all samples. Representatives of the Actinobacteria and Firmicutes phyla were predominant in the cultured bacterial communities. These communities were characterized by mesophilic and neutrophilic optima, as well as by the wide ranges of temperatures and pH suitable for metabolic activity. Moderate halotolerance in the presence of sodium or potassium chloride, as well as the high inhibitory effect of sodium hydrocarbonate and low inhibitory effect of magnesium sulfate, were revealed. The communities were highly resistant to the presence of 5% magnesium perchlorate in the culture medium. Strains resistant to antibiotics in the composition of the medium were revealed: The isolated strains were the most resistant to ampicillin, chloramphenicol, and cephalexin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Large territories of our planet are characterized by low temperatures. The World Ocean, most of which (90%) has the temperature below +5°C, occupies approximately 71% of the Earth’s surface (Hartmann, 2015). The polar regions, including the Antarctic continent and the cold zones of the Arctic, occupy about 20% of the Earth’s surface (Steven et al., 2006; Jansson and Taş, 2014). Ambient temperature is one of the most important parameters that control microbial activity in the natural environment. The temperature range for growth varies in different microorganisms: bacteria capable of growth and metabolic activity at around 0°C and lower temperatures were isolated from diverse natural and anthropogenic cold habitats (Rothschild and Mancinelli, 2001). The lower temperature limit for the metabolic activity of prokaryotes, known to date, is ‒18°C (Rivkina et al., 2000).
At temperatures below ‒20°C, the growth of prokaryotic cells is probably impossible due to its’ vitrification (Clarke, 2014). In this state, microorganisms can persists for a long time. Under conditions favorable for metabolism, they are able to become in metabolic active state. At the same time, thermophilic and hyperthermophilic prokaryotic species that can grow at +80°C or higher temperatures are known (Stetter et al., 1990).
An important biospheric function of permafrost is the cryopreservation of cells of various microorganisms (Bacteria, Archaea, and Fungi) in the state of metabolic dormancy (Rivkina et al., 1998). This is facilitated by the structural and mineralogical heterogeneity of heterophase media, such as soil and sedimentary rocks, in the horizontal and vertical directions, which results in the extremely high diversity of microlocal habitats in limited areas (Jansson and Taş, 2014).
Nucleotide sequences of the 16S rRNA gene, obtained from the Arctic and Antarctic soil samples, belong to bacteria of various phyla and functional groups (mainly anaerobic), including various methanogenic, sulfur-oxidizing, iron-reducing, and denitrifying bacteria. The bacterial diversity in permafrost rocks is significantly higher than that of archaea and fungi. Proteobacteria, Firmicutes, Chloroflexi, and Acidobacteria are the most typical phyla. Moreover, the metagenomic data obtained for permafrost soils and rocks often contain nucleotide sequences of the previously unknown phyla (Jansson and Taş, 2014). Analysis of total DNA revealed that the genes associated with various processes of carbon and nitrogen cycles, including those encoding chitinases, cellobiases, βрglycosidases, and β-galactosidases, were widespread in the microbial communities of permafrost soils. Genes associated with the processes of emission, transformation, and microbial degradation of greenhouse gases, such as CO2, CH4, and N2O, were also identified (Yergeau et al., 2010; Taş et al., 2014).
Phylogenetic analysis of the surface and underlying permafrost layers of the West Antarctic revealed two predominant groups belonging to the Proteobacteria and Actinobacteria phyla in all studied samples. According to the analysis of clonal libraries of total DNA from the ice sheet of Central Antarctica, the following genera predominated in all samples: Arthrobacter, Nocardioides, Bacillus, Caulobacter, Comamonas, Flavobacterium, Pseudomonas, and Sphingobacterium. Anaerobic denitrifying (2‒18 cells/g), methanogenic (2‒22 cells/g), and sulfate-reducing (102‒103 cells/g) bacteria were also identified (Gilichinsky et al., 2007).
Analysis of the surface soil of the Antarctic Dry Valleys by molecular genetic methods revealed the presence of Gammaproteobacteria (25%), Betaproteobacteria (9%), Firmicutes, Actinobacteria, and Bacteroidetes in the samples. Representatives of the genera Rhodococcus, Methylobacterium, Sphingomonas, and Bacillus (Goordial et al., 2016) were isolated from the same samples in pure cultures. The molecular genetic study of the taxonomic diversity of bacteria from the Antarctic soils (Yergeau et al., 2007; Zeng et al., 2013) indicated the presence of the genera Arthrobacter, Corynebacterium, Micrococcus, Brevibacterium, Bacillus, Pseudomonas, Achromobacter, Nocardia, Flavobacterium, Streptomyces, Alcaligenes, Chromobacterium, Aeromonas, and Planococcus (de los Ríos et al., 2004; Bulat, 2016).
In samples of snow collected near Druzhnaya and Leningradskaya Antarctic stations, the presence of bacteria (103 cells/mL) was shown by epifluorescence microscopy; 45.8% of them were metabolically active. Acinetobacter sp., Bacillus sp., Ochrobactrum sp., Pseudomonas sp., and Rhodococcus sp. were able to form macrocolonies on nutrient media at +4°C. Moreover, all isolates were capable of rapid growth at +37°C; therefore, a wide temperature range was suitable for their growth (Lopatina et al., 2013).
Among heterotrophic aerobic bacteria isolated from the soils of polar latitudes at low temperatures, representatives of the following genera were dominant: Arthrobacter, Corynebacterium, Cellulomonas, Microbacterium, and Brevibacterium. Their abundance sometimes reached 107 colony forming units (CFU) per gram of soil (Chattopadhyay, 2006).
Representatives of the Acetobacterium, Acinetobacter, Arthrobacter, Bacillus, Cellulomonas, Flavobacterium, Methanosarcina, Methylobacter, Micrococcus, Nitrobacter, Nitrosomonas, Pseudomonas, Rhodococcus, and Streptomyces prevail in the composition of the aerobic heterotrophic bacterial communities of the High Arctic soils. The total abundance of the cultured bacteria was approximately 4 × 107 cells/g of soil, and a direct relationship was found between microbial diversity and the amount of organic matter in the samples (Wagner et al., 2005).
In the soil samples collected in the Canadian High Arctic, bacteria of the phylum Firmicutes, known for their ability to form highly resistant spores, as well as bacteria of the Actinobacteria and Proteobacteria phyla, characterized by high resistance to desiccation (including cryogenic drying) and oxidative stress (Steven et al., 2008), were identified in the dominant positions.
The snow and ice surfaces, as well as the soil or mineral surface layers of glaciers outside the polar latitudes, are most affected by frequent fluctuations in temperature and water availability. Different authors (Foght et al., 2004; Hallbeck, 2009) reported bacterial abundance in the ice and snow surface layers of various glaciers in the range from 0.01 to 2.7 × 106 CFU/g (mL). The numbers of the reproductive cells in the modern and ancient surface ices aged up to 750 000 years were found to vary from <102 to 106‒107 CFU/g (Christner et al., 2003).
However, to date, the physiological characteristics of bacteria from cryo-arid ecosystems and their metabolic potential remains insufficiently studied. We studied cultured communities of aerobic heterotrophic bacteria isolated from the Antarctic surface and ancient frozen sedimentary rocks, as well as from the surface permafrost of the Severnaya Zemlya archipelago and their resistance to various physicochemical and biotic stress factors.
MATERIALS AND METHODS
The objects of this research were culturable bacteria isolated from the ancient permafrost sedimentary rocks of Antarctica and the surface permafrost of the Severnaya Zemlya archipelago.
The Permafrost sedimentary rock of the Antarctic desert (A-6/99 sample) was collected from borehole 6/99 drilled in the Beacon Valley lowlands (77°50′ S, 160°36′ E; at an altitude of 1270 m above sea level) from a depth of 1.3‒1.5 m. The age of the rocks was not more than 70 000 years (unpublished data from the Geological Survey of Canada). The permafrost sedimentary rock of the Antarctic Dry Valleys (Ho-3 sample) was collected from the borehole located at the mouth of the Taylor Valley (77°35′ S, 163°24′ E; at an altitude of 50 m above sea level) from a depth of 1.2‒1.5 m. The sample was described in detail in the study carried out by D.A. Gilichinsky et al. (2007). The surface permafrost of Komsomolets Island of the Severnaya Zemlya archipelago (Sz sample) was collected at the western tip of the island near the Yuny Strait (80°19′ N, 92°5′ E) from a depth of 0‒2 cm.
Prior to analysis, the samples were stored frozen at ‒20°C. The isolation of aerobic heterotrophic bacteria was performed using the glucose-peptone-yeast (GPY) medium containing various carbon sources and growth factors (Belov et al., 2018). The series of tenfold diluted soil suspension was directly plated on the solid nutrient media (Wood and Krieg, 1989). The inoculated dishes were incubated for 14 days at +10 and +25°С.
For the amplification of the 16S RNA gene, DNA preparations of the isolated cultures were obtained by boiling the two- or three-day-old biomass in a TAE buffer with subsequent homogenization using sterile glass beads (see complete protocol in Belov et al. (2019)). The target gene was amplified using the 27f + 1492r, 537r + 805r or 27f + 537r primer pairs, which are listed in the order of their use, if the PCR product could not be obtained with the preceding primer pair (see complete protocol in Belov et al. (2019)).
Resistance to stress factors was determined using liquid culture technique. Stress agents were added to the medium composition. To assess the resistance to temperature, the inoculated plates were incubated in thermostats at +2 and +4°С for 60 and 30 days, respectively, as well as at +10, +25, +37, +45, and +50°С for 10 days. Phosphate (pH 2‒7) and Tris-alkaline (pH 8‒12) buffer systems were used to estimate the tolerance to pH. Resistance to the presence of salts was determined at concentrations of 2, 5, 10, 15, and 20% (wt/vol) of sodium or potassium chlorides, sodium bicarbonate, and magnesium sulfate; magnesium perchlorate concentrations studied were 0.5, 1, 2, 5, 10, and 15% (wt/vol). Resistance to ampicillin, kanamycin, tetracycline, doxycycline, cephalexin, chloramphenicol, and rifampicin was studied on the media containing 100 μg/mL of one of these antibiotics. All tests were performed in triplicate with sterility controls. For all analyses, excluding low temperature cultivation, the incubation period was 10 days (Belov et al., 2018).
RESULTS
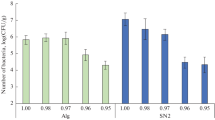
The samples were characterized by a high abundance of cultured cells. In the sample from the Antarctic desert (A6/99), close numbers of cultured bacteria were revealed under mesophilic and psychrophilic conditions: (2.4 ± 1.33) × 105 and (2.29 ± 1.25) × 105 CFU/g, respectively. In the sample of permafrost sedimentary rock from the Dry Valleys of Antarctica (Ho-3), the abundance of cells cultured at +25°C at the level of (5.27 ± 4.02) × 105 CFU/g and its decrease to (8.67 ± 1.24) × 102 CFU/g at +10°C were revealed. The surface permafrost collected on Komsomolets Island of the Severnaya Zemlya archipelago (Sz) was characterized by the following numbers of cultured bacteria: (1.93 ± 3.95) × 105 CFU/g at +25°С and (4.00 ± 3.53) × 105 CFU/g at +10°С (Fig. 1).
The cultured bacterial communities obtained in this study were characterized by moderate indices of bacterial colonies diversity. The most morphologically diverse culturable bacterial community was isolated from the Antarctic desert sample (36 morphological types of colonies). Twenty-eight morphotypes of bacterial colonies were isolated from the sample collected in the Dry Valleys, and twenty-six morphotypes of bacterial colonies were observed in the culturable bacterial community, which was isolated from the surface permafrost of Komsomolets Island (Fig. 2).
Gram-positive bacteria (representatives of the Actinobacteria and Firmicutes phyla) prevailed in all culturable communities: approximately 80% of colony forming units (Fig. 3). No connection between the community composition and the temperature of cultivation, or between the structure of the communities and the geographical location of the sampling point, was found.
Representatives of the Bacillus genus predominated in the culturable bacterial community isolated from the Antarctic desert sample at low temperatures (+10°C). Representatives of the genera Arthrobacter, Brevibacterium, Geodermatophilus, Leucobacter, and Pantoea, as well as of the species Dietzia cinnamea and Shinella kummerowiae, were also identified in this community. Bacteria of the Rhodococcus and Bacillus genera predominated in the communities isolated from this sample under mesophilic conditions. Representatives of the genera Brevibacterium, Leucobacter, Microbacterium, Paracoccus, Pseudoarthrobacter, and Streptomyces, as well as Gordonia terrae, Kocuria rosea, Leucobacter aridicollis, and Stenotrophomonas maltophilia, were identified.
Bacillus also predominated in the culturable bacterial community isolated from the ancient permafrost sample of the Dry Valleys of Antarctica at low temperatures (+10°C). Representatives of the genera Arthrobacter, Rhodococcus, and Streptomyces, as well as the species Arthrobacter oxydans, Bacillus simplex, Microbacterium barkeri, Planomicrobium glaciei, and Stenotrophomonas maltophilia, were also identified. Bacteria of the genus Bacillus (Bacillus infans and Bacillus sp.) were identified as dominants in the community growing at +25°C; Bacillus simplex, Bacillus subtilis, Lysinibacillus sphaericus, Paracoccus sp., Rhodococcus sp., and Streptomyces sp. were identified also in this community.
The cultured bacterial community isolated from the surface soil of the Komsomolets Island of the Severnaya Zemlya archipelago at low temperatures was characterized by the predominance of bacteria of the species Bacillus sp. and Microbacterium kitamiense (in equal proportions); Leucobacter aridicolis and Bacillus simplex were subdominant. Arthrobacter ginissoli and Arthrobacter sp. were also detected as the minor components. The community isolated from the same sample at +25°C was characterized by the predominance of bacteria of the species Micrococcus sp., Brevibacterium sediminis, Arthrobacter gisengisoli, and Leucobacter aridicolis. Arthrobacter sp., Bacillus pumilus, Bacillus simplex, Bacillus sp., Microbacterium paraoxydans, Microbacterium sp., Pedobacter sp., Rhizobium sp., Shingobacterium sp., and Stenotrophomonas maltophilia were isolated as minor components.
The high proportion of representatives of the genus Bacillus in all studied communities is noteworthy, as well as the isolation of representatives of the genera Arthrobacter, Dietzia, Geodermatophilus, Microbacterium, Pantoea, Planomicrobium, Shinella, and Stenotrophomonas only during low-temperature cultivation. Table 1 shows detailed data on the composition of cultured communities.
The studied bacterial communities were characterized by mesophilic values of the temperature optimum (+25°C), at which all isolated strains were able to reproduce, and by broad temperature ranges for their metabolic activity: from +2 to +50°C. Approximately 20% of the strains isolated from the Antarctic samples could reproduce at +2 and +4°С. At these temperatures, 26 and 32% of the cultures isolated from the permafrost of Severnaya Zemlya archipelago were able to proliferate in these conditions, respectively. The most thermotolerant properties were revealed for the cultures isolated from the frozen soils of the Dry Valleys of Antarctica: 36% of isolates reproduced at +50°С. The cultures isolated from the Antarctic desert and the permafrost of Severnaya Zemlya were less resistant to high temperatures: 10 and 11% of the studied cultures, respectively, reproduced at +50°C (Fig. 4).
The cultures studied had a neutrophilic optimum for growth and pH-tolerant features: the growth was observed in the pH range from 3 to 12. It is noteworthy that a high proportion (37% of isolates) of acidotolerant strains was detected in the cultured bacterial community, which was isolated from the soil of the Antarctic desert. In all studied communities, 20% of isolates retained their metabolic activity at pH 12 (Fig. 5).
On media supplemented with sodium or potassium chlorides, moderately halotolerant properties of bacteria isolated from the studied samples were found. The strains isolated from the soil of the Antarctic Dry Valleys were the least resistant to the presence of sodium or potassium chlorides: 56% of the strains were able to reproduce in the medium containing 2% NaCl. In the presence of 5% NaCl, 60% of the strains in the communities isolated from samples A6/99 and Sz were retaining their metabolic activity (Fig. 6). Potassium chloride had a less pronounced inhibitory effect on the studied strains (the average resistance to the presence of this salt was two times higher). In all the bacterial communities, strains that could reproduce in a saturated solution of this salt were identified (Fig. 7).
The presence of magnesium sulfate in the medium had a weak inhibitory effect on the studied communities: 60% of the isolates from the samples of the Antarctic desert and surface permafrost of Severnaya Zemlya, as well as 45% of the isolates from the frozen sedimentary rock of the Dry Valleys, retained their metabolic activity on the medium containing 20% magnesium sulfate (Fig. 8). The presence of low concentrations (2%, wt/vol) of sodium hydrocarbonate significantly inhibited all studied cultures. At the same time, less than 20% of the strains from all communities were able to reproduce on medium containing 5% sodium hydrocarbonate. Single strains, which were stable in the presence of 10% sodium hydrocarbonate, were identified in the culturable communities that were isolated from the permafrost of the Antarctic Desert and the Severnaya Zemlya permafrost (Fig. 9). The lowest resistance to the presence of magnesium perchlorate in the medium was shown by bacteria isolated from the permafrost of the Antarctic Dry Valleys, while the maximum resistance to this salt was found for isolates from the soil of the Antarctic desert. The metabolic activity of pure cultures isolated from all the studied samples was observed at a magnesium perchlorate concentration of up to 5% in the medium (Fig. 10).
Bacteria capable of reproduction on media containing antibiotics were identified in all cultured bacterial communities. High proportions of bacteria resistant to ampicillin, chloramphenicol or cephalexin in the studied communities were revealed. In addition to the resistance to the antibiotics listed above, strains that were resistant to tetracycline and kanamycin were found in all communities. In the communities isolated from the Antarctic frozen rocks, 10% of bacteria were resistant to doxycycline. Rifampicin-resistant bacterial strains were identified only in the cultured community isolated from the permafrost of the Antarctic Dry Valleys (Fig. 11).
DISCUSSION
The revealed numbers of the culturable bacteria indicate the enrichment of these samples with viable culturable bacterial cells both in the deep and in the surface layers. The observed numbers of culturable cells are in agreement with previous studies data (Christner et al., 2003; Foght et al., 2004; Hallbeck, 2009).
Close values for abundance of cells cultured at +10 and +25°C in the samples from the Antarctic desert and the surface permafrost of Komsomolets Island indicate widespread physiological adaptations in these bacterial communities to existence at low temperatures. At the same time, the discovery of a predominantly mesophilic development strategy of the community from the permafrost of the Dry Valleys of Antarctica can be interpreted as an adaptive mechanism to the seasonal cycles of this ecotope. The availability of water and, therefore, the possibility of metabolic processes in this region of Antarctica increase with increased temperatures due to the extremely low humidity of this region. The segment of the complex capable of in vitro reproduction at +10°C is likely to be more xerotolerant, and therefore able to reproduce at low moisture availability, while the metabolic activity of the main segment of the cultured community requires higher values of moisture availability, which can be reached in situ at higher ambient temperatures.
The diversity values obtained indicate the effects of environmental (rather than geographical) factors, which have determined the composition of the community. Close environmental conditions and close diversity of bacterial communities characterize the studied samples. The geographical location of the sampling site is therefore not the factor that determines the diversity of bacterial communities, although they were collected in the polar regions of different hemispheres.
The predominance of the Actinobacteria and Firmicutes phyla representatives in all studied communities can be explained by the ability of representatives of these phyla to switch to a state of metabolic dormancy, and their long-term preservation in a metabolically inactive state, as well as by the wide distribution of the psychrotolerant species and species resistant to oxidative stress among them (Steven et al., 2008). Moreover, similar results were previously obtained using molecular genetic methods (Steven et al., 2008, Jansson and Taş, 2014). The absence of obvious correlations between the composition of the communities and the temperature of cultivation, as well as between the structure of communities and the geographical location of the sample collection, additionally confirms the hypothesis of the predominant role of the ecological factor in the development of bacterial communities.
The prevalence of bacteria of the Bacillus genus in all studied communities may be explained by their ability to form spores that are highly resistant to various environmental stressful effects (Nicholson et al., 2000).
Bacteria of the Bacillus genus predominated in the communities isolated from the sample of the Antarctic desert (A6/99) under psychrophilic conditions (+10°C); representatives of the genera Arthrobacter, Brevibacterium, Geodermatophilus, Leucobacter, Pantoea, and Pseudoarthrobacter, as well as the species Dietzia cinnamea and Shinella kummerowiae were identified in the culture. Under mesophilic conditions (+25°C), representatives of the genera Rhodococcus and Bacillus prevailed in the cultured communities isolated from the same sample; bacteria of the Brevibacterium, Leucobacter, Microbacterium, Paracoccus, Pseudoarthrobacter, and Streptomyces genera, as well as the species Gordonia terrae, Kocuria rosea, Leucobacter aridicollis, and Stenotrophomonas maltophilia were determined in the culture.
Bacteria of the genus Bacillus also predominated in the cultured bacterial community isolated from the ancient permafrost of the Dry Valleys of Antarctica at low temperatures of cultivation (+10°C); representatives of the genera Arthrobacter, Rhodococcus, and Streptomyces, as well the species Arthrobacter oxydans, Bacillus simplex, Microbacterium barkeri, Planomicrobium glaciei, and Stenotrophomonas maltophilia, were identified. Bacteria of the genus Bacillus (Bacillus infans and Bacillus sp.) predominated in the community which was cultured at +25°C; Bacillus simplex, Bacillus subtilis, Lysinibacillus sphaericus, Paracoccus sp., Rhodococcus sp., and Streptomyces sp. were identified.
The cultured bacterial community isolated from the surface soil of Komsomolets Island of the Severnaya Zemlya archipelago at low temperatures was characterized by the predominance of bacteria of Bacillus sp. and Microbacterium kitamiense (in equal proportions); Leucobacter aridicolis and Bacillus simplex were identified as subdominant species. Arthrobacter ginissoli and Arthrobacter sp. were also determined in the culture. A community isolated from the same sample at a mesophilic temperature optimum (+25°C) was characterized by the predominance of bacteria of the species Micrococcus sp., Brevibacterium sediminis, Arthrobacter gisengisoli, and Leucobacter aridicolis; Arthrobacter sp., Bacillus pumilus, Bacillus simplex, Bacillus sp., Microbacterium paraoxydans, Microbacterium sp., Pedobacter sp., Rhizobium sp., Shingobacterium sp., and Stenotrophomonas maltophilia were isolated as minor components.
It is noteworthy that representatives of the genera Arthrobacter, Dietzia, Geodermatophilus, Microbacterium, Pantoea, Planomicrobium, Shinella, and Stenotrophomonas were identified only during low-temperature culturing, while they are also capable of growing at higher temperatures in axenic cultures.
Representatives of the genera from the studied samples were previously isolated from cryogenic and permafrost soils and rocks (Wagner et al., 2005; Bai et al., 2006; Bulat, 2006; Chattopadhyay, 2006; Zhang et al., 2007; Gilichinsky et al., 2008; Bull, 2011; Vishnivetskaya et al., 2011; Petrovskaya et al., 2012; Zhu et al., 2013; Suzina et al., 2015; Goordial et al., 2016; Singh et al., 2017; Efimenko et al., 2018; Sepehr et al., 2018; Humphrey et al., 2019). Moreover, the species Arthrobacter oxydans, Bacillus pumilus, Dietzia cinnamea, Leucobacter aridicollis, Microbacterium barkeri, Planomicrobium glaciei, and Stenotropomonas maltophilia, identified in the microbial communities from the studied frozen soils, were previously isolated from the soils of the hot arid Sahara, Gibson, and Mojave deserts (Belov et al., 2018; Belov et al., 2019).
Mesophilic and neutrophilic physiological profiles of the studied communities with wide range of metabolic activity were previously determined in the bacterial communities isolated from the soils of the arid Sahara (Egypt) and Gibson (Australia) deserts (Belov et al., 2018). We can therefore conclude that the tolerance of the metabolic state of bacterial cells (rather than the formation of specialized psychrophilic and thermophilic or acidophilic and alkalophilic subpopulations within the bacterial complex) is an adaptive strategy under the conditions of high stress load of various origins. The detection of a high proportion of thermotolerant strains in the sample collected in the Dry Valleys of Antarctica can be regarded as evidence of the preserving role of permafrost (Kryazhevskikh et al., 2012), and its capacity to preserve bacterial cells from past geological epochs.
The retention of metabolic activity of more than a quarter of the strains from all studied communities in the presence of 5% Mg(ClO4)2 is the most interesting result from the study of the resistance to various water-soluble salts. Antarctica and the microorganisms inhabiting various ecosystems of this continent are considered as the model objects in astrobiology studies (Gilichinsky et al., 2007), and the presence of oxidizing compounds in the regolith of various planets (in particular, the presence of perchlorates in the regolith of Mars), has been previously shown (Clark and Van Hart, 1981). The high resistance to in vitro presence of perchlorate can be interpreted as the possibility of reproduction of Earth-type bacteria in the regolith of Mars. However, due to the complex granulometric and mineralogical composition of the regolith, additional model experiments are required to clarify the effect of the presence of perchlorates in soils on bacterial communities.
On the one hand, the detection of antibiotic-resistant bacterial forms in the permafrost of polar ecosystems is additional evidence of the ecological role of antibiotics as signal metabolites of prokaryotes (Romero et al., 2011) and, on the other hand, it suggests that the permafrost bacterial communities are a reservoir of the determinants of antibiotic resistance. Therefore, these communities require further research: the study of the mechanisms of antibiotic resistance and the search for the producers of new antagonists (Andersson, 2003).
To summarize this research, the in vitro study of the bacterial communities from the cryo-arid soils of the Antarctic and Arctic revealed the polyextremotolerant features of the studied communities, indicating their potential ability to maintain metabolic activity in situ under aggressive environmental conditions. All studied communities were taxonomically diverse and dominated by representatives of Actinobacteria and Firmicutes phyla (representatives of the genera Bacillus, Rhodococcus, and Arthrobacter). The adaptive potential revealed in this study is quite interesting for the study of physiological mechanisms of anabiosis and involvement of these ecosystems in the global cycles of chemical elements in the biosphere, as well as for the development of new biotechnological processes.
LEGEND
A6/99 is a sample of permafrost sedimentary rock of the Antarctic desert.
Ho-3 is a sample of permafrost sediment rock, collected in the Antarctic Dry Valleys.
Sz is a sample of surface permafrost collected in the Komsomolets Island, Severnaya Zemlya archipelago.
REFERENCES
Andersson, D.I., Persistence of antibiotic resistant bacteria, Curr. Opin. Microbiol., 2003, vol. 6, no. 5, pp. 452–456.
Bai, Y., Yang, D., Wang, J., Xu, S., Wang, X., and An, L., Phylogenetic diversity of culturable bacteria from alpine permafrost in the Tianshan Mountains, northwestern China, Res. Microbiol., 2006, vol. 157, no. 8, pp. 741–751.
Belov, A.A., Cheptsov, V.S., and Vorobyova, E.A., Soil bacterial communities of Sahara and Gibson deserts: Physiological and taxonomical characteristics, AIMS Microbiol., 2018, vol. 4, no. 4, pp. 685–710.
Belov, A.A., Cheptsov, V.S., Vorobyova, E.A., Manucharova, N.A., and Ezhelev, Z.S., Stress-tolerance and taxonomy of culturable bacterial communities isolated from a central Mojave Desert soil sample, Geosciences, 2019, vol. 9, no. 4, p. 166. https://doi.org/10.3390/geosciences9040166
Bulat, S.A., Microbiology of the subglacial Lake Vostok: first results of borehole-frozen lake water analysis and prospects for searching for lake inhabitants, Philos. Trans. R. Soc., A, 2016, vol. 374, no. 2059, p. 20140292.
Bull, A.T., Actinobacteria of the extremobiosphere, Extremophiles Handbook, 2011, pp. 1203–1240.
Chattopadhyay, M.K., Mechanism of bacterial adaptation to low temperature, J. Biosci., 2006, vol. 31, no. 1, pp. 157–165.
Christner, B.C., Kvitko, B.H., and Reeve, J.N., Molecular identification of bacteria and eukarya inhabiting an Antarctic cryoconite hole, Extremophiles, 2003, vol. 7, no. 3, pp. 177–183.
Clark, B.C. and Van Hart, D.C., The salts of Mars, Icarus, 1981, vol. 45, no. 2, pp. 370–378.
Clarke, A., The thermal limits to life on Earth, Int. J. Astrobiol., 2014, vol. 13, no. 2, pp. 141–154.
Efimenko, T.A., Efremenkova, O.V., Demkina, E.V., Petrova, M.A., Sumarukova, I.G., Vasilyeva, B.F., and El’‑Registan, G.I., Bacteria isolated from Antarctic permafrost are efficient antibiotic producers, Microbiology, 2018, vol. 87, no. 5, pp. 692–698.
Foght, J., Aislabie, J., Turner, S., Brown, C.E., Ryburn, J., Saul, D.J., and Lawson, W., Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers, Microb. Ecol., 2004, vol. 47, no. 4, pp. 329–340.
Gilichinsky, D.A., Wilson, G.S., Friedmann, E.I., Mckay, C.P., Sletten, R.S., Rivkina, E.M., Vishnivetskaya, T.A., Erokhina, L.G., Ivanushkina, N.E., Kochkina, G.A., Shcherbakova, V.A., Soina, V.S., Spirina, E.V., Vorobyova, E.A., Fyodorov-Davydov, D.G., Hallet, B, Ozerskaya, S.M., Sorokovikov, V.A., Laurinavichyus, K.S., Shatilovich, A.V., Chanton, J.P., Ostroumov, V.E., and Tiedje, J.M., Microbial populations in Antarctic permafrost: biodiversity, state, age, and implication for astrobiology, Astrobiology, 2007, vol. 7, no. 2, pp. 275–311.
Gilichinsky, D., Vishnivetskaya, T., Petrova, M., Spirina, E., Mamykin, V., and Rivkina, E., Bacteria in permafrost, Psychrophiles: From Biodiversity to Biotechnology, Margesin, R., Schinner, F., Marx, J.C., and Gerday, C., Eds., Berlin, Heidelberg: Springer, 2008, pp. 83–102.
Goordial, J., Davila, A., Lacelle, D., Pollard, W., Marinova, M.M., Greer, C.W., DiRuggiero, J., McKay, C.P., and Whyte, L.G., Nearing the cold-arid limits of microbial life in permafrost of an upper dry valley, Antarctica, ISME J., 2016, vol. 10, no. 7, p. 1613.
Hallbeck, L., Microbial Processes in Glaciers and Permafrost. A Literature Study on Microbiology Affecting Groundwater at Ice Sheet Melting, Stockholm: Swedish Nuclear Fuel and Waste Management Co, 2009.
Hartmann, D.L., Global Physical Climatology, Newnes, 2015, vol. 103.
Humphrey, J., Seitz, T., Haan, T., Ducluzeau, A.-L., and Drown, D.M., Complete genome sequence of Pantoea agglomerans TH81, isolated from a permafrost thaw gradient, Microbiol. Resour. Announc., 2019, vol. 8, no. 1, p. e01486-18. https://doi.org/10.1128/MRA.01486-18
Jansson, J.K. and Taş, N., The microbial ecology of permafrost, Nat. Rev. Microbiol., 2014, vol. 12, no. 6, p. 414.
Kryazhevskikh, N.A., Demkina, E.V., Manucharova, N.A., Soina, V.S., Gal’chenko, V.F., and El’-Registan, G.I., Reactivation of dormant and nonculturable bacterial forms from paleosoils and subsoil permafrost, Microbiology, 2012, vol. 81, no. 4, pp. 435–445.
Lopatina, A., Krylenkov, V., and Severinov, K., Activity and bacterial diversity of snow around Russian Antarctic stations, Res. Microbiol., 2013, vol. 164, no. 9, pp. 949–958.
Nicholson, W.L., Munakata, N., Horneck, G., Melosh, H.J., and Setlow, P., Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments, Microbiol. Mol. Biol. Rev., 2000, vol. 64, no. 3, pp. 548–572.
Petrovskaya, L.E., Novototskaya-Vlasova, K.A., Spirina, E.V., Khokhlova, G.V., Rivkina, E.M., Gilichinsky, D.A., Dolgikh, D.A., and Kirpichnikov, M.P., Lipolytic enzymes of microorganisms from permafrost cryopegs, Dokl. Biol. Sci., 2012, vol. 445, no. 1, pp. 279–282.
de los Ríos, A., Ascaso, C., Wierzchos, J., Fernández-Valiente, E., and Quesada, A., Microstructural characterization of cyanobacterial mats from the McMurdo Ice Shelf, Antarctica, Appl. Environ. Microbiol., 2004, vol. 70, no. 1, pp. 569–580.
Rivkina, E., Gilichinsky, D., Wagener, S., Tiedje, J., and McGrath, J., Biogeochemical activity of anaerobic microorganisms from buried permafrost sediments, Geomicrobiol. J., 1998, vol. 15. no. 3, pp. 187–193.
Rivkina, E.M., Friedmann, E.I., McKay, C.P., and Gilichinsky, D.A., Metabolic activity of permafrost bacteria below the freezing point, Appl. Environ. Microbiol., 2000, vol. 66, no. 8, pp. 3230–3233.
Romero, D., Traxler, M.F., López, D., and Kolter, R., Antibiotics as signal molecules, Chem. Rev., 2011, vol. 111, no. 9, pp. 5492–5505.
Rothschild, L.J. and Mancinelli, R.L., Life in extreme environments, Nature, 2001, vol. 409, no. 6823, pp. 1092–1101.
Sepehr, S., Shahnavaz, B., Asoodeh, A., and Karrabi, M., Biodegradation of phenol by cold-tolerant bacteria isolated from alpine soils of Binaloud Mountains in Iran, J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng., 2019, vol. 54, no. 4, pp. 367–379.
Singh, P., Singh, S.M., Singh, R.N., Naik, S., Roy, U., Srivastava, A., and Bölter, M., Bacterial communities in ancient permafrost profiles of Svalbard, Arctic, J. Basic Microbiol., 2017, vol. 57, no. 12, pp. 1018–1036.
Stetter, K.O., Fiala, G., Huber, G., Huber, R., and Segerer, A., Hyperthermophilic microorganisms, FEMS Microbiol. Lett., 1990, vol. 75, no. 2–3, pp. 117–124.
Steven, B., Leveille, R., Pollard, W.H., and Whyte, L.G., Microbial ecology and biodiversity in permafrost, Extremophiles, 2006, vol. 10, no. 4, pp. 259–267.
Steven, B., Pollard, W.H., Greer, C.W., and Whyte, L.G., Microbial diversity and activity through a permafrost/ground ice core profile from the Canadian high Arctic, Environ. Microbiol., 2008, vol. 10, no. 12, pp. 3388–3403.
Suzina, N.E., Esikova, T.Z., Oleinikov, R.R., Gafarov, A.B., Shorokhova, A.P., Polivtseva, V.N., Ross, D.V., Abashina, T.N., Duda, V.I., and Boronin, A.M., Comparative characteristics of free-living ultramicroscopical bacteria obtained from extremal biotopes, Appl. Biochem. Microbiol., 2015, vol. 51, no. 2, pp. 159–168.
Taş, N., Prestat, E., McFarland, J.W., Wickland, K.P., Knight, R., Behre, A.A., Jorgenson, T., Waldrop, M.P., and Jansson, J.K., Impact of fire on active layer and permafrost microbial communities and metagenomes in an upland Alaskan boreal forest, ISME J., 2014, vol. 8, no. 9, p. 1904–1919.
Vishnivetskaya, T.A., Allan, J., Cheng, K., Chourey, K., Hettich, R.L., Layton, A., Liu, X., Murphy, J., Myky-tczuk, N.C., Phelps, T.J., Pfiffner, S.M., Saarunya, G., Stackhouse, B.T., Whyte, L., and Onstott, T.C., Microbial activity in active and upper permafrost layers in Axel Heiberg Island, AGU Fall Meeting Abstracts, 2011.
Wagner, D., Lipski, A., Embacher, A., and Gattinger, A., Methane fluxes in permafrost habitats of the Lena Delta: effects of microbial community structure and organic matter quality, Environ. Microbiol., 2005, vol. 7, no. 10, pp. 1582–1592.
Wood, W.A. and Krieg, N.R., Methods for general and molecular bacteriology, Washington, D.C.: Am. Soc. Microbiol., 1989.
Yergeau, E., Hogues, H., Whyte, L.G., and Greer, C.W., The functional potential of high Arctic permafrost revealed by metagenomic sequencing, qPCR and microarray analyses, ISME J., 2010, vol. 4, no. 9, pp. 1206–1214.
Yergeau, E., Newsham, K.K., Pearce, D.A., and Kowalchuk, G.A., Patterns of bacterial diversity across a range of Antarctic terrestrial habitats, Environ. Microbiol., 2007, vol. 9, no. 11, pp. 2670–2682.
Zeng, Y.H., Koblížek, M., Li, Y.X., Liu, Y.P., Feng, F.Y., Ji, J.D., Jian, J.C., and Wu, Z.H., Long PCR RFLP of 16S-ITS-23S rRNA genes: a high resolution molecular tool for bacterial genotyping, J. Appl. Microbiol., 2013, vol. 114, no. 2, pp. 433–447.
Zhang, G., Ma, X., Niu, F., Dong, M., Feng, H., An, L., and Cheng, G., Diversity and distribution of alkaliphilic psychrotolerant bacteria in the Qinghai–Tibet Plateau permafrost region, Extremophiles, 2007, vol. 11, no. 3, pp. 415–424.
Zhu, S., Zhao, Q., Zhang, G., Jiang, Z., Sheng, H., Feng, H., and An, L., Paracoccus tibetensis sp. nov., isolated from Qinghai-Tibet Plateau permafrost, Int. J. Syst. Evol. Microbiol., 2013, vol. 63, no. 5, pp. 1902–1905.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-34-00331) and by the Program of the Presidium of the Russian Academy of Sciences “Evolution of the Organic World and Planetary Processes” (Subprogram 1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by A. Panyushkina
Rights and permissions
About this article
Cite this article
Belov, A.A., Cheptsov, V.S., Vorobyova, E.A. et al. Culturable Bacterial Communities Isolated from Cryo-Arid Soils: Phylogenetic and Physiological Characteristics. Paleontol. J. 54, 903–912 (2020). https://doi.org/10.1134/S0031030120080043
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031030120080043