Abstract
Based on several modern plant species (Aristolochia clematitis, A. manshuriensis, Alliaria petiolata, Chloranthus japonicus, Symphytum officinale, Ambrosia trifida, Polemonium caeruleum, Plantago major, Populus tremula and P. × berolinensis), grown in botanical gardens of Moscow and Saint Petersburg, the stages of gametophyte and sporoderm development were compared. The most common regularities in the development of male gametophyte and its sporoderm were revealed. It is shown that the thicker the endexine, the later the intine starts to develop. The formation of tubular intine at the first mitosis of male gametophyte can occur after initiation of the intine, as it is observed in spores with endosporous spore germination in gametophyte. The dehydration of a pollen grain that is expressed in deposition of lipids and starch can start before the maturation of sporoderm that is observed in inaperturate pollen grains. Angiosperms show the broadest possibilities of variability in the sporoderm structure without losing the capacity for reproduction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The process of the sporoderm development from meiosis to the stage of mature pollen grain is described for a rather wide range of plants. The established main stages of the sporoderm development and their succession make it possible to compare the most different groups of subjects, for example, within the group of angiosperms. The microspore mother cells begins preparation for meiosis and form the specific callose wall, which separates sells from each other (Furness et al., 2002). After the meiosis, the callose wall continues to deposit on every microspore and develops conditions for specific arrangements for each daughter cell in a tetrad form (Matamoro-Vidal et al., 2015).
Under the callose the microspore begins to form the own sporoderm, first primexine and then, on its basis, ectexine. Then, endexine begins to form. After this, the callose is dissolved and microspores are released into a anther sack. This is the first significant change of the environment surrounding a microspore. During the free microspore (post-tetrad) period the mass deposition of sporopollenin of both tapetal and microsporongial origin on the marked ectexine of microspores begins. The vacuolation and devacuolation of the microspore is initiated and the innermost layer of the sporoderm—intine starts to develop. Then, asymmetric (endosporous) mitosis takes place and microspore transforms into male gametophyte—a pollen grain. The division of plant cells is always accompanied by the development of a cell wall. The first mitosis of male gametophyte is of no exception. A thin cell wall surrounds a generative cell and is indistinguishable in electron density and ultrastructure from the intine, surrounding a pollen grain as a whole. At the two-cell stage pollen grains of many angiosperms become mature (the termination of the development of sporoderm and the transition to the state of rest). Some plants are characteristic of three-cell pollen grains, but at this the appearance of sporoderm and the protoplast of a vegetative cell are similar to those in a mature two-cell pollen grain. As a rule, the hydratation of the pollen on a style and the pollen tube germination escape the attention of palynomorphologists. Therefore, let us consider that pollen grains (two-cell or three-cell) are released from anthers with mature sporoderm at the state of rest.
Thus, in the free microspore period one can distinguish much more observable states of the gametophyte stage, which can be compared with the stages of the sporoderm development. During our work we have compared the stages of the sporophyte and sporoderm development in several plant species from different taxonomic groups.
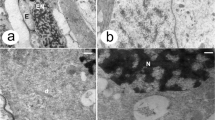
The vacuolation and devacuolation of the pollen grain Aristolochia clematitis occurs under the maximum development of the endexine. The two-cell stage of the development of a pollen grain of this species is accompanied by maximum development of the intine, dehydration of the protoplast of a vegetative cell and deposition of a large number of starch grains (Fig. 1c) (Polevova, 2015).
Stages of one-cell (vacuolation), two-cell and three-cell pollen grain in development of male gametophyte of several modern species of angiosperms, which are planted in botanical gardens of Moscow and Saint Petersburg. Transmission electron microscopy. (a) one-cell p.g. Ambrosia trifida Linnaeus, 1753; (b) one-cell p.g. Aristolochia manshuriensis Komarov, 1904; (c) two-cell p.g. Aristolochia clematitis Linnaeus, 1753; (d) one-cell p.g. Polemonium caeruleum Linnaeus, 1753; (e) two-cell p.g.; (f) three-cell p.g. Alliaria petiolate (Marschall Bieberstein) Cavara et Grande, 1913; (g) one-cell p.g. Chloranthus japonicus Siebold, 1829; (h) one-cell p.g.; (i) two-cell p.g. Symphythum officinalis Linnaeus, 1753; (j) one-cell p.g., (k, l) two-cell p.g. Plantago major Linnaeus, 1753; (m) one-cell p.g., (n) two-cell p.g. Populus × berolinensis Karl Koch, 1865; (o) two-cell p.g. Populus tremula Linnaeus, 1753. Designations: (V) vacuole, (G) generative cell, (S) spermium, (E) exine, (N) a core of vegetative cell; scale bar: 2 μm (a, c, d, h, k, l, m), 5 μm (b, e, g, n, o), 1 μm (i, j).
In another species Aristolochia manshuriensis, the vacuole coexists with maximum deposition of endexine, initiation of the intine and the presence of a small amount of storage starch (Fig. 1b). The two-cell pollen grain is characterized by maximally developed intine and abundant starch grains.
The vacuole resorption in Alliaria petiolata occurs simultaneously with nonequal mitosis and two-cell stage intersects with the vacuolation stage. The presence of a vacuole marks the development of a thin aperture plug and initiation of intine. The tubular intine continues to form at the two-cell stage (Fig. 1e) and reaches the maturation at the three-cell stage, when the protoplast of a vegetative cell is dehydrated (Fig. 1f) and a pollen grain is ready to release from an anther cavity.
The vacuolation and devacuolation of Chloranthus japonicus are characterized by the maximum development of endexine and initiation of the intine (Fig. 1g). The two-cell pollen grain accumulated the intine, and dehydrated, that is, it contains lipid drops and starch.
The vacuolation of Symphytum officinale is accompanied by the great development of the endexine and the aperture plug. The vacuole resorption is correlated with initiation of the intine (Fig. 1h). The two-cell stage is characterized by thickening of intine, especially around the aperture (Fig. 1i) (Gabaraeva et al., 2011).
The Ambrosia trifida vacuole coexists with the strong development of endexine (Fig. 1a). The vacuole is resorbed, whereas the intine starts to develop. The asymmetric mitosis already occurs in the absence of the vacuole (Gabaraeva et al., 2019; in press). The Polemonium caeruleum vacuole coexists with the aperture plug (Fig. 1d), and the intine forms much later (Grigorjeva and Gabaraeva, 2018).
The Plantago major vacuole coexists with the endexine and the aperture plug (Fig. 1j). The vacuole resorption occurs against the background of two-cell pollen grain and thin intine (Fig. 1k). Later, the thickness of the intine of a two-cell pollen grain increases and the protoplast of a vegetative cell is dehydrated (Fig. 1l) (Gabaraeva et al., 2016).
The two species of poplars Populus tremula and Populus berolinensis show that the vacuole coexists with the endexine and the initiation of intine (Fig. 1m). The two-cell pollen grain develops against the background of the vacuole resorption (Fig. 1n). Later, the intine continues to increase in thickness and lipid drops and starch grains are deposited in the protoplast (Fig. 1o).
The development of male gametophyte starts at the stage of meiosis being protected by a specific callose wall. Under the callose, independent primexine layers start depositing around each of microspores in a tetrad. The ectexine forms on the primexine becoming later enriched in the sporopollenin without significant difference in character. Before the dissolution of callose, the endexine starts developing. It forms the aperture plug and contributes to overcome degradation of callose. All these processes occur relatively fast and at this the microspore protoplast changes insignificantly. Due to these processes, only a cell volume increases and a cell shape becomes close to spheroidal.
After dissolution of the callose the significant events in the protoplast of the microspore and its shell take place:
(1) vacuolation and devacuolation of young microspore;
(2) asymmetric mitosis (formation of two-cell pollen grain);
(3) the second mitosis and formation of three-cell pollen grain;
(4) the emplacement of the intine and its increase in thickness, development of layers and channels in the intine;
(5) dehydration and deposition of starch grains and lipid drops (maturation of a pollen grain).
The above-listed processes occur in series or superimpose on one another, demonstrating own independence. There are only three logical structural constraints: vacuolation always precedes dehydration, asymmetric mitosis can not be ahead of the development of the intine, and the first mitosis precedes the second one.
The initiation of vacuolation and devacuolation in all pollen grains studied occurred at the maximally developed endexine. If the endexine is represented only by one lamella with a central white line, then the aperture plug determines the integrity of the exine. If the exine in the aperture (Alliaria petiolate and Chloranthus japonicus) or along the entire perimeter (Populus) is very thin, then the intine starts developing before the vacuole resorption and runs as usual before the first mitosis. The asymmetric mitosis, developing the microspore into pollen grain (male gametophyte), occurs at the vacuole resorption in case of the thin endexine or at a total absence of the vacuole in species with the well-defined endexine. At this, the ectexine can be rather thick as in Alliaria petiolate and Plantago major or thin as in poplars.
The dehydration of pollen grains is usually accompanied by the accumulation of starch grain and/or lipid drops in the protoplast of a vegetative cell. However, the storage starch in the inaperturate pollen grains of Aristolochia manshuriensis and Populus × berolinensis begins to appear in the protoplast at the presence of incompletely resorbed vacuole. However, lipid drops usually occur together with pollen grains, which have to develop under cold stress.
When considering the processes of formation of different layers of microspore shell, then one can note that many structural problems can be resolved by a simple change of relative duration of these processes. Thus, depositing the innermost layer—intine—at the stage of the vacuole formation, there is no need to develop a thick endexine and it will be enough to have a small aperture plug. In this case, the intine maintains the integrity of sporoderm and the shape of a pollen grain. The thicker the endexine, the later the intine starts to develop. The first asymmetric mitosis is not highly dependable on the initiation of intine. The intine often starts to develop still at the stage of one-cell microspore that is demonstrated by endosporous microspore germination producing male gametophyte. Such succession of processes is characteristic of the germination of microspores at heterospory of not only spermaphytes, but also sporophytes. Some mosses experience the first mitoses inside the sporoderm even in isospores (Alfayate et al., 2013, Ignatov et al., 2016). Dehydration of a pollen grain, expressed in the deposition of lipids and starch, can start long before the maturation of the sporoderm that is observed, however, only for inaperturate pollen grains. Moreover, the pollen grains of Aristolochia manshuriensis with mature sporoderm were not found that does not exclude seed reproduction of this species. Flowering plants show wide possibilities of variability of the sporoderm structure without losing the capability for reproduction.
REFERENCES
Alfayate, C., Ron, E., Estébanez, B., and Pérez-Batista, M.A., Mature spores of four pleurocarpous mosses in the Canary Islands: ultrastructure and early germination stages, Bryologist, 2013, vol. 116, no. 2, pp. 97–112.
Furness, C.A., Rudall, P.J., and Sampson, F.B., Evolution of microsporogenesis in angiosperms, Int. J. Plant Sci., 2002, vol. 163, no. 2, pp. 235–260.
Gabaraeva, N., Grigorjeva, V., and Polevova, S., Exine and tapetum development in Symphytum officinale (Boraginaceae). Exine substructure and its interpretation, Plant Syst. Evol., 2011, vol. 296, nos. 1–2, pp. 101–120.
Gabarayeva, N., Gabarayeva, V., Polevova, S., and Hemsley, A.R., Pollen wall and tapetum development in Plantago major l. (Plantaginaceae): assisting self-assembly, Grana, 2016, vol. 56, no. 2, pp. 1–31.
Grigorjeva, V.V. and Gabarayeva, N., Pollen wall ontogeny in Polemonium caeruleum (Polemoniaceae) and suggested underlying mechanisms of development, Protoplasma, 2017, vol. 255, no. 1, pp. 109–128.
Ignatov, M.S., Ignatova, E.A., Fedosov, V.E., Ivanov, O.V., Ivanova, E.I., Kolesnikova, M.A., Polevova, S.V., Spirina, U.N., and Voronkova, T.V., Andraeaobrium macrosporum (Andreaeobryopsida), Arctoa, 2016, vol. 25, pp. 1–51 [in Russian, with additional data on its morphology].
Matamoro-Vidal, A., Prieu, C., Furness, C.A., Albert, B., and Gouyon, P.-H., Evolutionary stasis in pollen morphogenesis due to natural selection, New Phytol., 2016, vol. 209, no. 1, pp. 376–394.
Polevova, S.V., Ultrastructure and development of sporoderm in Aristolochia clematitis (Aristolochiaceae), Rev. Palaeob. Palyn., 2015, vol. 222, pp. 104–115.
ACKNOWLEDGMENTS
I am deeply grateful to N.R. Meier-Melikyan, N.I. Gabaraeva, V.V. Grigorieva, Ya.V. Kosenko, M.V. Tekleva, and O.V. Volkova for possibility to get acquainted with the stages of sporoderm development of many plant species.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-04-00971a).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by D. Voroshchuk
Rights and permissions
About this article
Cite this article
Polevova, S.V. The Stages of Gametophyte and Sporoderm Development in Pollen Grains. Paleontol. J. 53, 795–798 (2019). https://doi.org/10.1134/S0031030119080173
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031030119080173