Abstract
The structure and electrical and optoelectronic properties of nanostructured carbon films obtained by methane plasma deposition with subsequent annealing have been studied. It is shown that the film formation conditions affect the final physicochemical parameters. The film morphology has been investigated by atomic force microscopy, scanning electron microscopy, Raman spectroscopy, X-ray energy-dispersive analysis, and analysis of the current–voltage characteristics (CVCs). The film thicknesses range from 20 to 150 nm at the carbon-to-oxygen (C/O) atomic ratio of 4 : 1. Structural studies show that the films obtained consist of nanographite flakes with the lateral dimensions in the range from 5 to 12 nm and contain different fractional concentrations of sp3/sp2 crystalline phases of carbon. It is established that the structural quality of carbon films decreases with an increase in the annealing temperature from 650°C to 800°C. At the same time, the degree of graphitization increases, which is indicated by Raman spectroscopy data and sheet resistances calculated from the CVCs. Photocurrents are calculated from the temperature dependences of the CVCs; it is found that the samples exhibit photosensitivity in the temperature range from room temperature to –173°C. These results may be useful for designing day and night light sensors and temperature sensors operating in a wide temperature range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In view of the intense industrial and technological development of Far North territories, there is an increasing need for electronic sensors (including light and temperature ones) operating at low temperatures [1]. These devices can be based on various carbon modifications (graphene, nanotubes, amorphous carbon, etc.). For example, graphene has unique optical characteristics and high carrier mobility [2]. The unresolved technological problems of the use of graphene and carbon nanotubes (CNTs) in scalable production of electronic devices limit their range of application [2, 3]. Nanostructured carbon films (nsCFs) with different fractional concentrations of diamond and graphite (sp3/sp2) carbon phases can successfully compete with the above materials when designing temperature and optical sensors due to the technological efficiency of production [4–6]. Carbon phases in different spn hybridization states determine the final physical and chemical properties of a material [7]. For example, the graphite-like carbon (GLC) phase is characterized by a more ordered structure and a high electrical conductivity [8, 9]. In contrast, the diamond-like carbon (DLC) phase is characterized by high optical transparency and mechanical stability [10–12]. It was confirmed experimentally that nanocarbon coatings formed by plasma-enhanced chemical vapor deposition (PECVD) on the surface of solid-state substrates are temperature sensitive [13–18]. Carbon films containing nanographene flakes were investigated in [19]; they exhibited a high ratio of the generated photocurrent to the incident light power (0.35 A/W).
In this paper, we report the results of studying the optical and electrical properties of nanographite flakes obtained using standard technological processes, including plasma deposition and annealing.
EXPERIMENTAL
Nanostructured carbon films were formed by methane (CH4) plasma deposition with subsequent annealing, which was described in detail in [20]: the power of a high-frequency (13.56 MHz) ETNA-100-PT inductive plasma source (Zelenograd, Russia) was 200 W and the deposition time of carbon atoms on the surface of silicon substrates was up to 9 min. The sample heating temperature in the reaction chamber did not exceed 50°C. The chamber was preliminarily evacuated to about 10–4 mbar. The CH4 flow rate was 30 cm3/min. The working pressure in the chamber after the gas inlet was about 0.0045 mbar. In the second stage of the synthesis, the samples were annealed in argon (Ar) at a temperature Tann of 650–800°C for 15 to 45 min.
The film structures were studied using atomic force microscopy (AFM) (NTegra Spectra, Zelenograd, Russia), scanning electron microscopy (Oxford Instruments, United Kingdom), and Raman spectroscopy (Ntegra Spectra, Zelenograd, Russia). Raman spectra were excited by laser radiation with the beam diameter of 1 μm and the wavelength of 532 nm. The elemental composition was determined by X-ray energy-dispersive spectroscopy (XEDS) (JEOL-7800F, Japan). The temperature dependences of the current–voltage characteristics (CVCs) were measured on an ASEC-03 setup (Zelenograd, Russia). Electrical contacts were formed using silver paste. The contacts were deposited on surfaces of the films formed and dried at a temperature of 120°C for 20 min. The photosensitivity was analyzed using a 35‑W halogen lamp with the total luminous flux of 490 Lm.
RESULTS
To determine thicknesses of the synthesized samples, scratches with a width up to several tens of micrometers were made by a diamond cutter; as a result, carbon film strips were removed from the substrate surface. The heights of the formed steps (corresponding to the thicknesses of the formed carbon films) were measured by AFM at the film–substrate interface. The results obtained show that the films have a thickness from 20 to 150 nm. The film thickness depends on both the plasma deposition conditions (plasma power and processing time) and the annealing parameters (temperature and duration). Figure 1 shows the 3D profile of the film surface (P = 200 W, 9 min) formed upon annealing at 650°C for 15 min. An increase in the annealing temperature and time reduces the thickness of the film until it is removed completely.
Figure 2a presents a SEM image of a scratch on the carbon film surface. The regions denoted in the figure as “Spectrum 1” and “Spectrum 2” correspond to the carbon film and the substrate, respectively. Using the JEOL-7800F XEDS analyzer attached to the SEM setup, it was found that the films contain mainly carbon atoms and a small amount of oxygen (Fig. 2b). As expected, the composition in the scratch region corresponds to the SiO2 substrate.
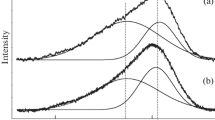
It was shown in our previous study [21] that a film corresponding to an amorphous hydrogenated carbon (a:C–H) film with a wide photoluminescence band from 1000 to 3300 cm–1 is formed in the first synthesis stage (carbon deposition in methane plasma). After the second synthesis stage (annealing), the Raman spectra clearly exhibit the D (~1355 cm–1) and G (~1590 cm–1) peaks typical of graphite-containing structures [22–24]. The D peak intensity depends on the structural imperfection and vibrational intensity of sp3 hybridized carbon, whereas the G peak intensity is related to breathing vibrations of sp2 hybridized carbon in hexagonal graphene cells [22]. Note that the Raman spectra depend on the annealing conditions. Figure 3 shows the Raman spectra for the samples annealed at temperatures of 700 and 750°C for the same time (30 min) and at 800°C for 15 min. It was found that an increase in the annealing temperature increases the total intensity ratio for the D and G peaks (ID/IG) from 1.7 to 3.5 (Table 1). This effect may be due to a decrease in the structural quality of graphene cells and/or a change in the sp3/sp2 ratio of crystalline phases of carbon in the film structure [22, 25]. The latter is favored by the shift of the G peak maximum from 1590 to 1582 cm–1 with an increase in the annealing temperature, which is related to the increase in the fractional concentration of the sp3 phase in the carbon film [22–24].
The lateral sizes of sp2 graphite nanocrystallites [22–24] can be estimated from the found total intensity ratios for the D and G peaks using the formula proposed in [24]:
where λ is the laser source wavelength. The estimations showed that the lateral sizes La of nanographite domains decrease from 12 nm (T = 650°C) to 5 nm (T = 800°C) (Table 1). This decrease in La may also cause a change in the fractional concentrations of the sp2 and sp3 crystalline phases in the formed carbon films [23, 24].
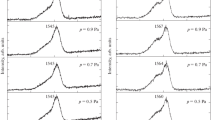
The sheet resistances of the carbon films in the temperature range of 80–300 K were determined from the temperature dependences of the linear CVCs (Fig. 4a). As is shown in Fig. 4b, an increase in the annealing temperature from T = 650°C to T = 750°C in the second stage of film synthesis reduces the relative resistance Ri/R0, where R0 is the film resistance after the annealing at T = 650°C and Ri is the resistance after annealing at different temperatures. Note that the resistance slightly increases after the annealing at 800°C.
It follows from the results obtained that the increase in the electrical conductivity (up to T = 750°C) is accompanied, as was shown above, by a decrease in the sizes of nanographite flakes. In this case, the increase in the electrical conductivity may be due to more efficient formation of nanocrystalline carbon, as was demonstrated by Ferrari et al. [22]. One can suggest that the thus formed films mainly consisting of nanographite and graphene flakes will have a more homogeneous structure with a relatively high electrical conductivity. At the same time, the increase in the resistance at T = 800°C may be related to a decrease in the thickness of the formed films, which is accompanied by a decrease in the structural quality. Note that, when the annealing time increases to 30 min (T = 800°C), the initial amorphous carbon films are completely removed from the substrate surface.
To study the photosensitivity of the films, a bias voltage of 8 V was applied to the measuring probe contacts. Changes in the current upon switching the light source on and off were recorded. The time of the c-arbon film reaction to the illumination change was ~0.1 s. Photocurrents Iph were calculated according to the formula
where Il is the current strength under illumination and Id is the current strength in the absence of illumination. Figure 5 shows the temperature dependences of Iph. As can be seen, the Iph value increases from ~80 μA at the measurement temperature T = 100 K to ~180 μA at T = 250 K. The highest photocurrent was observed after the annealing at 750°C in the second stage of the synthesis.
The photosensitivity and photoelectric quantum yield were estimated using the total luminous flux of 490 lm from a halogen lamp with a maximum at ~550 nm. To a first approximation, calculations yield the photosensitivity of ~0.3 μA/W and the quantum yield of ~10–3. The observed photosensitivity of the obtained carbon films can be explained by the light absorption in graphite and graphene nanocrystals formed during the annealing.
CONCLUSIONS
Nanostructured carbon films obtained by methane plasma deposition with subsequent annealing at a temperature in the range of 650–800°C were investigated. The AFM measurements revealed that the formed films were 20 to 150 nm thick. The Raman spectra showed that the main peaks of the carbon films are typical of graphite-containing structures. The lateral sizes of sp2 graphite nanocrystallites were estimated from the position and the total intensities of the main D and G peaks to be from 5 to 12 nm, depending on the synthesis conditions. In addition, it was established that the ratio of the fractional concentrations of sp3/sp2 hybridized carbon in the structures obtained increases with an increase in the temperature of annealing of the initial a:C–H films. The temperature dependences of the photocurrents were also investigated; it was revealed that the photoresponse of the formed carbon films is retained in the temperature range from room temperature to ~173°C. Thus, the results obtained suggest that the presented nanostructured carbon films can be applied as temperature sensors and day and night light sensors in Arctic and Far North territories.
REFERENCES
Yu. Zeng, T. Li, Yo. Yao, T. Li, L. Hu, and A. Marconnet, Adv. Funct. Mater. 29, 1901388 (2019). https://doi.org/10.1002/adfm.201901388
D. Neumaier, S. Pindl, and M. C. Lemme, Nat. Mater. 18, 525 (2019). https://doi.org/10.1038/s41563-019-0359-7
Z. Wang, H. Gao, Q. Zhang, Yu. Liu, J. Chen, and Z. Guo, Small 15, 1803858 (2019). https://doi.org/10.1002/smll.201803858
N. Aslan, J. Mater. Sci.: Mater. Electron. 32, 16927 (2021). https://doi.org/10.1007/s10854-021-06254-1
D. Yu, K. Goh, H. Wang, L. Wei, W. Jiang, Q. Zhang, L. Dai, and Yu. Chen, Nat. Nanotechnol. 9, 555 (2014). https://doi.org/10.1038/nnano.2014.93
V. D. N. Bezzon, T. L. A. Montanheiro, B. R. C. De Menezes, R. G. Ribas, V. A. N. Righetti, K. F. Rodrigues, and G. P. Thim, Adv. Mater. Sci. Eng. 2019, 4293073 (2019). https://doi.org/10.1155/2019/4293073
M. N. Drozdov, Yu. N. Drozdov, A. I. Okhapkin, P. A. Yunin, O. A. Streletskii, and A. E. Ieshkin, Tech. Phys. Lett. 46, 290 (2020). https://doi.org/10.1134/s1063785020030190
V. I. Khristolyubova, Vestn. Kazanskogo Tekhnologicheskogo Univ. 18 (12), 114 (2015).
X. Li, S. Deng, M. N. Banis, K. Doyle-Davis, D. Zhang, T. Zhang, J. Yang, R. Divigalpitiya, F. Brandys, R. Li, and X. Sun, ACS Appl. Mater. Interfaces 11, 32826 (2019). https://doi.org/10.1021/acsami.9b06442
M. Saleem, Kh. S. Karimov, Z. M. Karieva, and A. Mateen, Phys. E: Low-dimensional Syst. Nanostruct. 43, 28 (2010). https://doi.org/10.1016/j.physe.2010.06.011
D. K. Rajak, A. Kumar, A. Behera, and P. L. Menezes, Appl. Sci. 11, 4445 (2021). https://doi.org/10.3390/app11104445
T. Kasiorowski, J. Lin, P. Soares, C. M. Lepienski, C. A. Neitzke, G. B. De Souza, and R. D. Torres, Surf. Coat. Technol. 389, 125615 (2020). https://doi.org/10.1016/j.surfcoat.2020.125615
V. Kumar, A. A. Bergman, A. A. Gorokhovsky, and A. M. Zaitsev, Carbon 49, 1385 (2011). https://doi.org/10.1016/j.carbon.2010.12.004
A. Muthuraja, S. Naik, D. K. Rajak, and C. I. Pruncu, Diamond Relat. Mater. 100, 107588 (2019). https://doi.org/10.1016/j.diamond.2019.107588
T. Chunjaemsri, E. Chongsereecharoen, N. Chanlek, P. Kidkhunthod, H. Nakajima, S. Tunmee, R. Yimnirun, and S. Rujirawat, Radiat. Phys. Chem. 176, 109073 (2020). https://doi.org/10.1016/j.radphyschem.2020.109073
B. Huang, L.-T. Liu, S. Han, H.-M. Du, Q. Zhou, and E.-G. Zhang, Diamond Relat. Mater. 129, 109345 (2022). https://doi.org/10.1016/j.diamond.2022.109345
B. Paramanik and D. Das, Appl. Surf. Sci. 579, 152132 (2022). https://doi.org/10.1016/j.apsusc.2021.152132
V. S. Jatti, Int. J. Thin Film Sci. Technol. 11, 233 (2022). https://doi.org/10.18576/ijtfst/110211
X. Zhang, Z. Lin, D. Peng, and D. Diao, Nanomaterials 9, 327 (2019). https://doi.org/10.3390/nano9030327
E. P. Neustroev, A. R. Prokopyev, S. O. Semenov, V. I. Popov, F. F. Protopopov, A. S. Andreev, N. A. Savvinova, and E. S. Lukin, IOP Conf. Ser.: Mater. Sci. Eng. 1079, 042086 (2021). https://doi.org/10.1088/1757-899x/1079/4/042086
E. P. Neustroev and A. R. Prokopiev, Fiziko-Khim. Aspekty Izucheniya Klasterov, Nanostruktur Nanomaterialov 11, 629 (2019). https://doi.org/10.26456/pcascnn/2019.11.629
A. C. Ferrari and J. Robertson, Philos. Trans. R. Soc. London. Ser. A 362, 2477 (1824). https://doi.org/10.1098/rsta.2004.1452
E. F. Sheka, Ye. A. Golubev, and N. A. Popova, Nanomaterials 10, 2021 (2020). https://doi.org/10.3390/nano10102021
L. G. Cançado, K. Takai, T. Enoki, M. Endo, Y. A. Kim, H. Mizusaki, A. Jorio, L. N. Coelho, R. Magalhães-Paniago, and M. A. Pimenta, Appl. Phys. Lett. 88, 163106 (2006). https://doi.org/10.1063/1.2196057
W. J. Yang, Yo. Choa, T. Sekino, K. B. Shim, K. Niihara, and K. H. Auh, Mater. Lett. 57, 3305 (2003). https://doi.org/10.1016/s0167-577x(03)00053-3
Funding
This study was supported by the Ministry of Science and Higher Education of the Russian Federation (project no. FSRG-2022-0011) and the grant of the Head of the Republic of Sakha (Yakutia) for Support of Young Scientists, Researchers, and Students “Formation of Carbon-Containing Film Coatings Deposited on Various Surfaces in Methane Plasma.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Sin’kov
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prokopev, A.R., Neustroev, E.P. Nanostructured Carbon Films Obtained by CH4 Plasma Deposition and Annealing at High Temperatures: Structural Features and Their Influence on the Electrical and Optoelectronic Properties. Opt. Spectrosc. 131, 1231–1236 (2023). https://doi.org/10.1134/S0030400X24700218
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X24700218