Abstract
The spectral-luminescent properties of europium(III) complexes with o-methoxybenzoic acid and phosphorus-containing neutral ligands with an island structure and the compositions Eu(MOBA)3 · 3H2O and Eu(MOBA)3 · L, where MOBA is o-methoxybenzoic acid and L is hmpa (hexamethylphosphortriamide, tppo (triphenylphosphinoxide), and Et6pa (hexaethylphosphortriamide), are studied. It is found that the excitation energy transfer to the europium ion occurs from the levels of both o-methoxybenzoic acid and phosphorus-containing neutral ligands. The electronic absorption and luminescence excitation spectra, as well as the Stark structure of the 5D0−7Fj ( j = 0–2) electronic transitions in the luminescence spectra of mixed-ligand complexes of europium(III) o-methoxybenzoates, are analyzed. The highest luminescence intensity is demonstrated by europium(III) methoxybenzoate with triphenylphosphinoxide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Photostable coordination compounds of rare-earth elements with carbonic acids and nitrogen- and phosphorus-containing neural ligands, as well as light-transforming polymer materials based on them, can be used in various molecular electronic devices, optoelectronics, and organic light-emitting diodes and lasers [1–7]. The advantages of luminescent aromatic lanthanide carboxylates are high chemical and thermal stability, radiation stability, and the possibility of changing their composition, structure, and physicochemical properties by varying the ligand coordination. Promising compounds are mixed-ligand europium(III) complexes with o-methoxybenzoic acid. For example, mixed-metal terbium–yttrium o-methoxybenzoate with 1,10-phenanthroline was successfully used as a component of light-emitting layers in electroluminescent devices [8]. Optimization of the spectroscopic characteristics of rare-earth compounds by changing fragments of their crystal structure is an important stage in the fabrication of efficient luminescent lanthanide compounds, which can be used to synthesize optical materials.
The spectral-luminescent properties of europium compounds with methoxybenzoic acids and nitrogen-containing neutral ligands have been studied [9–14], but complex compounds of europium with phosphorus-containing neutral ligands have not been synthesized.
In the present work, we report the results of investigation of the composition, structure, and thermal properties of mixed-ligand europium(III) complexes with o-methoxybenzoic acid and phosphorus-containing neutral ligands with the composition Eu(MOBA)3 · L, where MOBA is the o-methoxybenzoic acid anion and L is hexaethylphosphortriamide (Et6pa), hexamethylphosphortriamide (hmpa), and triphenylphosphinoxide (tppo).
EXPERIMENTAL
To synthesize mixed-ligand europium compounds with o-methoxybenzoic acid and phosphorus-co-ntaining neutral ligands, we used the following pure-grade agents: six-water europium(III) chloride, o‑methoxybenzoic acid, phosphorus-containing ligands (hexaethylphosphortriamide, hexamethylphosphortriamide, and triphenylphosphinoxide). o‑Methoxybenzoic acid was recrystallized from 90% ethanol.
Europium(III) o-methoxybenzoates with phosphorus-containing neutral ligands were prepared as follows: to 1 mmol of six-water europium(III) chloride dissolved in a minimum amount of distilled water 3 mmol of o-methoxybenzoic acid and 1 mmol of a neutral phosphorus-containing ligand dissolved in 15 mL of 90% ethanol were added. The solution PH was increased to 5.5–6.0 using 10% ammonia solution, and the mixture was allowed to stand for precipitation. The precipitate was filtered, washed with a water–ethanol mixture, and dried in air. The obtained europium(III) complexes were fine-grained yellow powders insoluble in most nonpolar solvents.
The luminescence excitation and luminescence spectra were measured on an RF-5301 Shimadzu spectrometer.
The electronic absorption spectra were measured sing an RF-2550 Shimadzu spectrometer. The compound concentration in 96% ethanol was 10–4 mol/L.
RESULTS AND DISCUSSION
The measured electronic absorption spectra of the synthesized europium(III) coordination compounds with o-methoxybenzoic acid and phosphorus-containing neutral ligands are presented in Fig. 1. As is seen, the complexation leads to a change in the position of the band corresponding to the π–π* transition of o-methoxybenzoic acid (λmax = 225–235 nm). The obtained mixed-ligand europium(III) o-methoxybenzoates are characterized by a more complex shape of the electronic absorption spectra than o-methoxybenzoic acid. The electronic absorption spectra of the synthesized mixed-ligand o-methoxybenzoates with phosphorus-containing neutral ligands exhibit additional absorption lines typical for coordinated phosphorus-containing neutral ligand molecules. In particular, the spectra of Eu(MOBA)3 ⋅ tppo clearly exhibit three absorption bands of tppo with λmax = 240–276 nm. The absorption band of o-methoxybenzoic acid in the electronic absorption spectra of this complex compound is shifted to longer wavelengths by 4‒5 nm from its position in the spectrum of free o‑methoxybenzoic acid at λmax = 290 nm corresponding to the singlet (Sππ*) transition [15, 16]. The absorption spectra of neutral ligands (hexamethylphosphortriamide and hexaethylphosphortriamide) and o-methoxybenzoic acid in the absorption spectra of the Eu(MOBA)3 · hmpa and Eu(MOBA)3 · Et6pa compounds overlap in the range of 220–235 nm.
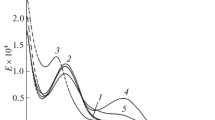
The luminescence excitation spectra of mixed-ligand europium(III) coordination complexes with o‑methoxybenzoic acid and phosphorus-containing neutral ligands consist of a broad high-frequency band composed of the absorption bands of coordinated ligand and the narrow lines of the f–f transitions of europium(III) ions (Fig. 2). The luminescence excitation spectra of the mixed-ligand o-methoxybenzoates synthesized by us contain the bands of the ππ* transitions of o-methoxybenzoic acid and the corresponding phosphorous-containing ligand, which leads to energy transfer from both the triplet levels of o-methoxybenzoic acid and the levels of the neutral phosphorus-containing ligand to europium.
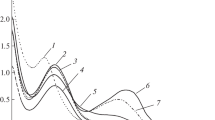
The new synthesized mixed-ligand europium(III) o-methoxybenzoates exhibit red luminesce (in solid state and solution) at both room temperature and 77 K (Fig. 3). The general character of luminescence sp-ectra in the series of synthesized europium(III) o‑methoxybenzoates with phosphorus-containing neutral ligands is retained. The most intense bands in the luminescence spectra of the europium(III) complex compounds belong to the 5D0–7F2 electric dipole transition. This transition is sensitive to substitution of the neutral ligand in the mixed-ligand complexes, namely, one observes redistribution of intensities of individual lines and changes in the Stark splitting structure of the 7F2 level. The luminescence spectra of the synthesized new mixed-ligand complexes in the region of the 5D0–7F2 electric dipole transition contain up to three components. The 5D0–7F1 magnetic dipole transition intensity is almost three–five times lower than the intensity of the bands of the 5D0–7F2 electric dipole transition. Splitting of the two edge bands of the 5D0–7F1 magnetic dipole transition considerably changes with substitution of the neutral phosphorus-containing ligand, which indicates the that it enters the coordination sphere of the europium(III) ion. The bands of the 5D0–7F4 electric dipole transition are quite pronounced in the luminescence spectra. They are comparable in intensity with the band of the 5D0–7F1 magnetic dipole transition.
We determined the integral intensities of the 5D0‒7F2 transition of europium(III) ions for the s-ynthesized mixed-ligand europium(III) complexes. It was found that the europium luminescence intensity in the complex compounds with phosphorus-containing compounds is higher than in europium(III) o‑methoxybenzoate hydrate. Europium(III) o‑metho-xybenzoate with triphenylphosphinoxide is characterized by the maximum luminescence intensity due to the strong donor properties of the triphenylphosphinoxide neutral ligand, the existence of a more developed π-conjugated system of efficient ultraviolet light absorbers, and the absence of water molecules in the coordination sphere of the complex compound.
Comparative analysis of the luminescence intensity of the mixed-ligand europium(III) o-methoxybenzoate complexes with phosphorus-containing neutral ligands and the previously studied o-methoxybenzoates with nitrogen-containing neutral ligands (we synthesized and studied the luminescent properties of europium o-methoxybenzoates with 1,10-phenanthroline and 2,2-dipyridyl) shows that the luminescence intensity of compounds with phosphorus-containing neutral ligand is 10–20% higher.
Thus, we have synthesized new luminescent mixed-ligand europium(III) o-methoxybenzoates with phosphorus-containing neutral ligands. It is shown that excitation energy transfer to europium ions occurs from the levels of both o-methoxybenzoic acid and neutral phosphorus-containing ligands. The electronic absorption and luminescence excitation spectra and the Stark structure of the 5D0−7Fj ( j = 0–2) electronic transitions in the luminescence spectra of europium(III) o-methoxybenzoate complexes are analyzed.
REFERENCES
J. C. G. Bunzli, and S. V. Eliseeva, Chem. Sci. 4, 1913 (2013).
K. Binnemans, Handbook on the Physics and Chemistry of Rare Earths (Elsevier, Amsterdam, 2005), Vol. 35, p. 107.
Y. Hasegawa and T. Nakanishi, RSC Adv. 5, 338 (2015).
J. Zhang, R. Wang, J. Bai, and S. Wang, J. Rare Earths 20, 449 (2002).
J. Zhang, R. Wang, and H. Yang, Chin. J. Anal. Chem. 31, 472 (2003).
H. Kataoka, T. Kitano, T. Takizawa, Y. Hirai, T. Nakanishi, and Y. Hasegawa, J. Alloys Compd. 601, 293 (2014).
Hui-Juan Sun, Ai-Ling Wang, Hai-Bin Chu, and Young-Liang Zhao, Luminescence, No. 30, 131 (2015).
Y. Shi, Z. Deng, J. Xiao, D. Xu, Z. Chen, and R. Wang, J. Lumin. 122–123, 272 (2007).
R. Wang, L. Li, L. Jin, and S. Lu, J. Rare Earths 16, 445 (1998).
X. Li, Z. Bian, L. Jin, S. Lu, and S. Huang, J. Mol. Struct. 522, 117 (2000).
X. Li and Y. Q. Zou, Z. Kristallogr. New Cryst. Struct. 218, 445 (2003).
Z. H. Gao, H. Wang, J. Y. He, and R. F. Wang, Acta Crystallogr., E 65, 1240 (2009).
R. X. Ma, Z. M. Chen, Z. H. Gao, S. P. Wang, R. F. Wang, and J. J. Zhang, Synth. Met. 159, 1272 (2009).
L. Jin, R. Wang, L. Li, S. Lu, and S. Huang, Polyhedron 18, 487 (1998).
I. V. Kalinovskaya, V. E. Karasev, and A. N. Pyatkina, Russ. J. Inorg. Chem. 44, 380 (1999).
I. V. Kalinovskaya, A. N. Zadorozhnaya, and V. E. Karasev, Zh. Fiz. Khim. 34, 1175 (1987).
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation (state order no. 0265-2014-0001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Translated by M. Basieva
Rights and permissions
About this article
Cite this article
Kalinovskaya, I.V. Spectral-Luminescent Properties of Mixed-Ligand Europium(III) Complexes with O-Methoxybenzoic Acid and Phosphorus-Containing Neutral Ligands. Opt. Spectrosc. 128, 698–701 (2020). https://doi.org/10.1134/S0030400X20060077
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X20060077