Abstract
We present the results of an investigation into the interaction of SF6 molecules and clusters in a molecular beam with resonant IR laser radiation at different stages of the beam evolution along the axis of its propagation. The beam has been formed as a result of gas-dynamic expansion of a mixture of SF6 with argon carrier gas during expansion from a pulsed nozzle. The experimental setup and the investigation method are described. It has been shown that selective vibrational excitation of SF6 molecules with a specific sulfur isotope by a CO2 laser near the nozzle edge causes suppression of the clustering process of these isotopic molecules. Selective IR excitation of clusters under the conditions of the formed cluster beam leads to isotopically selective dissociation of clusters. Depending on the experimental conditions including different distances of the irradiation zone of particles from the nozzle edge, the results of measuring the efficiency and selectivity of molecular clustering suppression and cluster dissociation processes are presented. It has been shown that both of these processes make it possible to achieve high selectivity values for the 32S and 34S sulfur isotopes. In the case in which the clustering of SF6 molecules was selectively suppressed, selectivity values α ≥ 25–30 have been obtained. Upon selective dissociation of (SF6)2 dimers under similar expansion conditions of the gas mixture, selectivity values α ≥ 20–25 for 32SF632SF6 dimers with respect to 34SF632SF6 dimers have been obtained. Particular attention has been paid to measurements at a high dilution of SF6 in argon under conditions of predominant formation of (SF6)mArn mixed clusters. The potential of using studied processes as a basis for the technology of the laser isotope separation are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
At present, investigations on the development of low-energy laser isotope separation methods in molecules remain very important [1–13]. The development of the method of selective multiphoton IR dissociation of molecules, which is well known [14–17] and which has been successfully applied in practice to the separation of carbon isotopes [18–21], in the case of uranium isotopes, is considerably impeded due to a relatively high energy consumption of the process, the absence of powerful laser systems, and a number of other factors [1]. Thus, to dissociate a UF6 molecule, it is necessary to absorb about 40–45 IR photons with a wavelength of 16 μm; i.e., the energy absorption per molecule is about 3.1–3.5 eV.
One way to develop the low-energy laser isotope separation methods is to use physical and chemical processes, the activation energy of which does not exceed 0.3–0.5 eV [1–13]. Such activation energies are specific to physicochemical processes of adsorption and desorption of molecules on surfaces, including the surface of large clusters, as well as for the processes of dissociation and fragmentation of weakly bound van der Waals molecules [1].
Among low-energy laser isotope separation methods with the use of IR lasers, the method of isotope-selective suppression of clusterization of molecules applying IR lasers at the nozzle exit upon gas-dynamic expansion and the method of isotope-selective IR dissociation of small molecular van der Waals clusters are considered to be the most promising. These approaches were demonstrated for the first time in [22]. We note that we successfully used the former of these methods for the bromine-isotope-selective control of clustering of CF3Br molecules both with each other [23] and with argon atoms [24]. The selectivity values obtained in these studies were relatively small; however, the results of these experiments showed that, first, the method of isotopically selective suppression of clustering can be successfully applied to molecules with a small isotope shift of laser-excited vibrations (for CF379Br and CF381Br isotopomers, its value is correct value is 0.124 cm–1) and, second, in the case of suppression of clustering of CF3Br molecules with argon atoms, it is possible to achieve higher values of the selectivity than in the case of suppression of clustering of CF3Br molecules with each other.
This paper presents the results of our experiments on isotopically selective suppression of clusterization and selective dissociation of clusters with SF6 molecules with a natural content of sulfur isotopes. The isotopic shift in SF6 is much larger than in the case of CF3Br, which allowed us to expect to achieve significantly higher selectivity values. For this purpose, special attention was paid to experiments with a large dilution of SF6 in argon, at which (SF6)mArn mixed clusters predominantly form.
1 EXPERIMENTAL SETUP AND INVESTIGATION METHOD
1.1 Experimental Setup
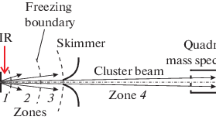
The setup (Fig. 1) involves two high-vacuum chambers, one of which contains a pulsed molecular-cluster beam source, and the other contains a quadrupole mass spectrometer (QMS). The upper limit of the registration range of mass numbers of the mass spectrometer was m/z = 300 amu. As an ion detector, a VEU-6 secondary emission electronic multiplier was used in the QMS. The chambers of the molecular beam source and the QMS were separately evacuated by two turbomolecular pumps. The QMS was controlled with a PC. To excite molecules and clusters in the jet, we used a frequency-tunable cw CO2 laser. The setup also contained a pulse synchronization system and a data acquisition and processing system.
An SF6 molecular cluster beam was generated in the source chamber by the gas-dynamic cooling of a gas mixture of molecules under study with an argon carrier gas due to supersonic expansion through a General Valve modified pulsed nozzle [25], the diameter of the exit hole of which was d = 0.16 or 0.25 mm. The pulse repetition rate of the nozzle was 1 Hz. Depending on the pressure and gas composition above the nozzle, the duration of the nozzle opening pulse was varied from 0.3 to 1.6 ms (at half-height). The gas pressure above the nozzle was varied in the range P0 = 130–300 kPa. A Beam Dynamics (Model 1) skimmer (with a hole diameter of 0.49 mm), located at a distance of 50 mm from the nozzle, cut out a molecular/cluster beam from the central part of the supersonic flow produced by this nozzle. The thus formed beam entered the ionization chamber of the QMS. The distance from the nozzle edge to the ionization chamber of the QMS was 570 mm.
A discretely tunable cw CO2 laser was used in experiments. The laser power can be varied between 13–15 W. Using copper mirrors and a NaCl focusing spherical lens with a focal length of f = 110 mm, the IR radiation of the laser was introduced through a NaCl window into the chamber with the molecular cluster beam. The diameter of the IR spot at the focal point of the lens was ≈0.5 mm. The laser beam intersected the molecular-cluster beam at an angle of 90°. The beam could be displaced along the axis of the molecular cluster beam with a movable translation stage. To vary the laser power, radiation attenuators were used. A maximum laser radiation power introduced into the chamber was about 10 W, which corresponds to the radiation intensity of ≈5 KW/cm2. The tuning of the CO2 laser to particular lasing lines was monitored with an optoacoustic cell filled with ammonia. The ammonia IR absorption lines served as references for tuning the frequency of the CO2 laser.
1.2 Investigation Method
Figure 1 schematically shows key regions of formation of a cluster beam upon gas-dynamic expansion of a gas mixture close to the nozzle edge, in which particles were irradiated by a CO2 laser. Upon gas-dynamic expansion at the nozzle edge, several stages can be distinguished [23, 25, 26] that proceed in different regions of the jet (Fig. 1).
Region 1 is characterized by rapid cooling of translational and internal degrees of freedom of molecules accompanied by the energy transfer to the kinetic energy of the directed motion of the flow and by the medium transition to the supersaturated state, which leads to the formation of seed clusters.
Collisional region 2 in which, on the one hand, the gas-dynamic cooling of molecules is continued and, on the other hand, the process of growth of clusters in the jet proceeds with some heating of the system due to the condensation energy.
Region 3 lies behind the “freezing” boundary in which a transition to the collisionless motion of particles and stabilization of the cluster system occurs.
Region 4 is located between the skimmer and the QMS, and beam particles fly freely in it.
To study the influence of the resonant IR excitation of jet molecules on the formation of the cluster beam at different stages of clustering process, particles in the corresponding regions of the jet on the path of the flow were irradiated and changes in the beam parameters in the detection region were registered. The signal of the cluster component can decrease for several reasons [23, 25, 26].
In region 1, a local increase in temperature due to IR excitation of molecules can be realized, which will prevent the formation of seeds. In the ideal, this may prevent the further clustering of molecules. Under conditions of a strong dilution of molecules by the inert gas, when the probability of collisions of molecules with each other is small, one can selectively suppress the clustering of molecules of only excited type (certain isotopomer).
The IR laser irradiation of particles in region 2 results in the vibrational heating of molecules and clusters that were formed in the jet by this moment. In this case, a partial fragmentation of clusters is possible. Particles are heated simultaneously with their competing gas-dynamic cooling (especially, in the presence of a carrier gas) and, also, with some further change in the cluster composition of the jet (growth of clusters, evolution of the size distribution).
Upon irradiation of particles in region 3, in which there are no collisions, the action of the IR radiation is mainly reduced to the heating of clusters and to their fragmentation, which manifests itself in a corresponding decrease in the signal of the cluster component of the beam. Therefore, depending on the particular position of the jet irradiation region, the excitation of jet particles by the resonant IR laser radiation in regions 1–3 considered above can result, either in the suppression of clustering of molecules or in the dissociation of formed clusters.
Selectivity α of the laser control over the clustering of molecules was measured as follows. The value of selectivity α was determined based on the measurements of the ion signals of SF6SF\(_{5}^{ + }\) and SF5Ar+ cluster fragments from homogeneous and mixed clusters in the beam, as well as of the SF\(_{5}^{ + }\) ion signal, a significant contribution to which is made by fragmentation of clusters. In experiments, we measured the fraction contribution q = (q1, q2, q3) to the ion signal to be detected from each of the three sulfur isotopes, 32S, 33S, and 34S, with respect to its initial natural ratio. The measured mass peaks were fitted by a Gaussian function, then, the values of q were determined. The values of the selectivity upon excitation of the ith isotopomer of SF6 molecules with respect to the jth isotopomer were defined as α(i/j) = (1 – jq)/(1 – iq). Such a determination of the selectivity implies that the formation of different isotopic modifications of clusters, in particular, dimers, occurs statistically. A similar technique was also used to determine the selectivity of dissociation of clusters (see below).
We note that, in this work, in the process of selective dissociation of clusters, because of a low natural concentration of 33S (0.75%), we mainly studied the change in the ratio between the 32SF6 (95%) and 34SF6 (4.2%) isotopomers.
2 RESULTS AND DISCUSSION
Experiments were performed using argon as a carrier gas. In preliminary measurements with an SF6/Ar mixture, the working pressure range of the mixture above the nozzle and the degree of the gas dilution were selected, which were found to be P0 = 130–220 kPa and SF6/Ar = 1/80–1/200, respectively.
In studies of processes of isotopically selective suppression of the clustering of molecules and dissociation of van der Waals clusters, it is necessary to precisely adjust the position the laser beam with respect to the nozzle edge. This circumstance is illustrated in Fig. 2, which shows the dependence of the relative (laser on/laser off) value of the SIR/S0 cluster signal (with respect to the 32SF632SF\(_{5}^{ + }\) peak) on the distance of the particle irradiation zone (laser spot) from the nozzle edge, which was obtained upon irradiation of particles by the 10P(14) laser line (at the frequency of 949.48 cm–1). An SF6/Ar gas mixture at a pressure ratio of 1/100 and a total pressure of P0 = 133 kPa was used above the nozzle. This laser generation line is in rather good resonance both with the absorption spectrum of free 32SF6 molecules [27] and with the spectrum of the high-frequency absorption band of (32SF6)2 dimers [28–30].
Dependence of the relative value of the SIR/S0 cluster signal (with respect to the peak from 32SF632SF\(_{5}^{ + }\) ions) on the position of a laser beam (the images of the nozzle edge and laser spot are scaled). The irradiation was performed by line 10P(14) of a CO2 laser (949.48 cm–1) at a radiation power of 9 W. An SF6/Ar = 1/100 mixture at a total pressure of P0 = 133 kPa was used.
Figure 2 shows the size of the laser spot and also the position of the nozzle head. The ion peak at 273 amu was taken as a cluster signal, which corresponds to the 32SF632SF\(_{5}^{ + }\) ion fragment of the (SF6)2 dimer. A dip near the nozzle edge can be clearly seen in the dependence presented in Fig. 2. At the minimum, the signal from this line falls to ~40% of the initial value (without irradiation of the jet). As the distance from the nozzle increases, the signal is partially restored (to ≈80%) and then remains unchanged. The width of the dip is ≈1.4 mm, or five to six nozzle calibers (diameters) (in this experiment, a nozzle with a hole diameter of d = 0.25 mm was used). The signal in the range of negative displacements corresponds to the contact of the laser beam with the nozzle edge. The observed dip corresponds to the region of prevention (suppression) of the clustering of SF6 molecules as a result of their vibrational excitation [23, 25, 31]. As the distance of the irradiation region of particles from the nozzle increases, we pass to the region of developed and “frozen” condensation; therefore, a decrease in the ion signal in this region is mainly related to the dissociation of clusters by the IR laser radiation [23, 25, 31].
Therefore, in the course of the preliminary experiments presented above, conditions were found under which it is possible to realize the sulfur-isotope-selective action on the processes of clusterization and dissociation of clusters.
2.1 Selective Suppression of Clustering of Molecules
The frequency of the laser IR radiation has a significant influence on the process of suppressing the clustering of SF6 molecules both between themselves and with argon atoms. In experiments, particles in a molecular beam were excited using two lines of the CO2 laser: 10P(16) (at a frequency of 947.74 cm–1) and 10P(34) (at a frequency of 931.00 cm–1)—curves 1 and 2, respectively, in Fig. 3a. The former of these two lines is in good resonance with 32SF6 molecules [27], while the latter is resonant with 34SF6 molecules [32].
(a) Dependences of a normalized signal from 32SF632SF\(_{5}^{ + }\) ions on the distance of the irradiation region from the nozzle edge at different IR radiation frequencies: (1) line 10P(16) (947.74 cm–1) and (2) line 10P(34) (931.00 cm–1). (b) Dependences of a normalized signal from (1) 32SF632SF\(_{5}^{ + }\) and (2) 34SF632SF\(_{5}^{ + }\) ions on the distance of the irradiation region from the nozzle edge upon irradiation on line 10P(34) (931.00 cm–1). The radiation power was 5.8 W; an SF6/Ar = 1/200 mixture at a total pressure of P0 = 200 kPa was used.
Figure 3a presents the dependences of the value of the 32SF632SF\(_{5}^{ + }\) dimeric ion signal on the distance of the particle irradiation region from the nozzle edge (the beam cross section dimension at the focus is shown at the bottom of the figure). It can be seen that the measured curves behave significantly differently. On curve 1, for the laser beam position close to the nozzle edge (where it is still possible to act on free molecules), the signal from 32SF632SF\(_{5}^{ + }\) dimers is suppressed almost completely. As the laser beam is displaced away from the nozzle edge, the value of this signal is restored almost to the initial level. The strongest decrease in the cluster signal is observed upon resonant excitation of molecules near the boundary of the nozzle edge, where molecules are still free (clusters have not yet been formed). With increasing distance from the nozzle, condensation of molecules begins, the spectrum of formed particles comes out of resonance, and the dimeric signal is restored. (SF6)2 dimers practically do not absorb the IR radiation from the laser at the 10P(16) line.
Another pattern is observed if the radiation at the 10P(34) laser line, which is resonant with 34SF6 molecules, is used for the excitation [32]. The excitation of this isotope component near the nozzle edge under the conditions of these experiments hardly should affect the 32SF632SF\(_{5}^{ + }\) signal because of the selectivity of the process, which is indeed the case. At the same time, the 10P(34) line falls into the range of the absorption band of the 32SF632SF6 dimer [29]. Therefore, a decrease in the corresponding ion signal is explained by the dissociation of dimers, which are increasingly formed with increasing distance of the excitation region of particles from the nozzle. The attainment of saturation of the 32SF632SF\(_{5}^{ + }\) signal can be related either with the insufficient laser radiation power or with the contribution from clusters of larger sizes to this signal.
The dependence on the distance for the ion signal from another isotopic modification, namely, 34SF632SF\(_{5}^{ + }\), upon irradiation at the 10P(34) line is significantly different from the dependence for the 32SF632SF\(_{5}^{ + }\) ion. This is clearly demonstrated by Fig. 3b (cf. the upper and lower curves). In this case, the laser excites 34SF6 molecules and, correspondingly, upon irradiation of particles near the nozzle edge, the formation of 34SF632SF6 dimers is suppressed.
It is significant that, for all products, the characteristic spatial length of the “dip” (see Fig. 3a) is about 0.4–0.5 mm, which is approximately equal to the diameter of the laser beam. Therefore, the length of the region in which the clustering of molecules is suppressed most efficiently is apparently no more than two to three nozzle calibers.
Passing to the quantitative characteristics of the process of selective prevention of clustering, it should be noted that, apart from the tuning accuracy of the CO2 laser to lines, the frequency of which is resonant with vibrations of corresponding isotopomers, as well as the accurate spatial localization of the IR radiation caustic with respect to the nozzle edge, the value of the selectivity is also affected by the radiation power of the CO2 laser. In particular, experiment shows that the selectivity of clustering suppression process decreases with increasing power of the IR radiation. Thus, upon suppression of clustering of SF6 molecules with each other, the selectivity dropped from a maximum value of α ≈ 20 at a laser power of P ≈ 2 W to α ≈ 3 at P ≈ 8 W.
It should be noted that we succeeded in obtaining even greater selectivity in the process of preventing the clustering of SF6 molecules with argon atoms upon the formation of (SF6)mArn mixed clusters under conditions of strong dilution of the mixture above the nozzle. Figure 4 shows the fragment of the mass spectrum in the range of 168 amu, which contains the Ar32SF\(_{5}^{ + }\) and Ar34SF\(_{5}^{ + }\) ion peaks without IR irradiation (open circles) and upon irradiation at the 10P(16) CO2 laser line, which is in resonance with 32SF6 molecules (closed circles).
Ar32SF\(_{5}^{ + }\) and Ar34SF\(_{5}^{ + }\) ion peaks: (open circles) initial signal in the absence of the IR irradiation and (closed circles) signal after the IR excitation on line 10P(16) of a CO2 laser. The radiation power was 7.2 W; an SF6/Ar = 1/200 mixture at a total pressure of P0 = 200 kPa was used.
Figure 4 clearly shows the distortion of the natural ratio between sulfur isotopes as a result of the IR excitation: the calculated value of fraction contribution q to the ion signal for 32S, 33S, and 34S isotopes is q = (0.56, 1, 1), which means that there is no action on 34SF6. This formally corresponds to an infinitely high selectivity of the process of preventing the clustering of SF6 molecules with argon atoms; however, taking into account the instrumental error, we believe that the selectivity in this experiment is α ≥ 25–30.
2.2 Selective Dissociation of Clusters
To realize selective dissociation of (32SF6)2 dimers, we used the 10P(34) laser line (with the frequency at 931.00 cm–1) in order to eliminate and/or reduce the probability of excitation of 32SF6 monomers on this line. The frequency of this line is considerably tuned away from the center of the absorption band of 32SF6 molecules [27] but coincides well with the low-frequency absorption band of (32SF6)2 dimers [28–30]. An SF6/Ar mixture was used at a pressure ratio of 1/200 and at a total gas pressure above the nozzle of P0 ≈ 163 kPa. At such a pressure, a significant fraction of (32SF6)2 dimers is present in the beam. Initially, in test experiment, the jet was irradiated near the nozzle exit, where the number of dimers should be minimal. As should be expected, in this case, no selective dissociation of dimers was observed.
Upon irradiation of the jet at a distance of 2 mm from the nozzle edge (in this experiment, a nozzle with a diameter of d = 0.16 mm was used), i.e., in the region where clusterization was completed to a large extent, we observed a distortion of the natural isotope ratio between the signals from 32SF632SF\(_{5}^{ + }\) and 34SF632SF\(_{5}^{ + }\). In this case, we can introduce selectivity parameter α as a ratio of loss β of the corresponding isotopic components of the 32SF632SF6 and 34SF632SF6 dimers: α = β(32, 32S)/β(34, 32S), β(i, jS) = 1 – i, jS/i, jS0, where i, jS and i, jS0 are the values of the corresponding ionic dimer signals after the irradiation of particles and before their irradiation, respectively. Then, at a distance of 2 mm from the nozzle edge, the selectivity is α ≈ 2.
The selectivity of dissociation of 32SF632SF6 clusters with respect to 34SF632SF6 clusters manifests itself much more clearly in the case when particles are irradiated in the “frozen” beam region far from the nozzle edge and when the clustering process is totally completed. This follows from Fig. 5, which shows the mass spectra of the SF6SF\(_{5}^{ + }\) dimeric ion fragment with its isotopic modifications without irradiating the jet and when the jet is irradiated at the 10P(34) laser line (931.00 cm–1) at a distance of 7 mm from the nozzle.
32SF632SF\(_{5}^{ + }\) and 34SF632SF\(_{5}^{ + }\) ion peaks: (open circles) initial signal in the absence of the IR irradiation and (closed circles) signal after the IR excitation on line 10P(34) of a CO2 laser. The radiation power was 4.4 W; an SF6/Ar = 1/200 mixture at a total pressure of P0 = 163 kPa was used.
As can be seen from Fig. 5, a rather considerable (more than 20%) decrease in the 32SF632SF\(_{5}^{ + }\) ion signal is observed, while the 34SF632SF\(_{5}^{ + }\) ion signal remains almost unchanged within the statistical error (the calculated value of fraction contribution q to the ion signal for 32S, 33S, and 34S isotopes is q = (0.88, 1, 1)). Taking into account the measurement error, the estimated value of the dissociation selectivity of 32SF632SF6 dimers relative to 34SF632SF6 dimers is α ≥ 20–25.
In the end of this section, we point out the factors that determine the isotopic selectivity upon preventing clusterization and dissociation of clusters. To ensure the effective excitation of molecules containing target isotopes, first of all, a high optical selectivity is necessary. If we will proceed from the data on the width and the shape of the IR absorption spectra of SF6 isotopomers at a low (about 50 K) temperature [33] and a small (about 60 MHz) radiation width of the CO2 laser, we can state that the optical excitation selectivity of 32SF6 molecules with respect to 34SF6 molecules at a frequency of 947.74 cm–1 (at the 10P(16) laser line) is αexc(32S/34S) > 102.
The selectivities of the process of suppression of clustering of molecules that we obtained are considerably smaller than this value. In our opinion, the following factors could be the reasons for the decrease in the selectivity. First, because of a high concentration of molecules in the excitation region, an efficient vibrational–vibrational energy exchange between 32SF6 and 34SF6 molecules may occur. It is likely that this is the main factor of the loss of selectivity in the process of control over the clustering of molecules. Second, in the region of excitation of molecules near the nozzle exit, the jet still remains to be rather hot, and, therefore, the temperature of particles can be considerably higher than 50 K, and, consequently, the optical selectivity of the excitation of particles will be smaller than the value indicated above. In addition, because the size of the laser spot in our experiments is rather large (≈0.5 mm in diameter), the irradiation region of particles significantly exceeds the region of localization of the maximum selectivity of molecule excitation with respect to both their concentration and their temperature. In the jet that rapidly expands in space and in time, these parameters vary strongly even within the limits of the irradiation region itself.
The factors listed above should not affect the process of selective dissociation of clusters due to the action on a much more homogeneous medium with a steady-state concentration of particles and temperature. From this point of view, the process of selective dissociation of clusters has an advantage over the process of suppression of clustering. However, broader absorption spectra of clusters (due to the occurrence of the size distribution of clusters) compared to monomers in a cold molecular jet can lower the optical selectivity, especially in the case of small isotopic shifts in laser-excited vibration.
3 CONCLUSIONS
We investigated processes of isotopically selective suppression of the clustering of SF6 molecules with Ar atoms upon gas-dynamic expansion of an SF6–Ar mixture and dissociation of (SF6)mArn van der Waals clusters under the action of resonant IR radiation from a CO2 laser.
It was found that both of these processes allow achieving high values of the selectivity with respect to 32S and 34S sulfur isotopes. Thus, in the case of selective suppression the clustering of SF6 molecules with argon atoms using the SF6/Ar = 1/200 mixture, the selectivity values α ≥ 25–30 were obtained. Upon selective dissociation of (SF6)2 dimers under similar effusion conditions of the mixture, slightly lower selectivity values were obtained: α ≥ 20–25 for 32SF632SF6 dimers with respect to 34SF632SF6 dimers.
A detailed analysis of the condensation process of molecules upon their gas-dynamic expansion from the nozzle allows to reveal certain advantages and disadvantages inherent in the examined processes as applied to the possibility of their use as a basis of isotope separation technology, as well as to compare them with each other. Upon suppression of clustering, resonant IR radiation affects the region of the beam that contains cold molecules, which potentially ensures high optical excitation selectivity. At the same time, the spatial dimension of the region in which optimal conditions for the excitation of molecules are realized is quite small, as a result of which the region of irradiation of particles in our experiments (≈0.5 mm in diameter) exceeded the region of localization of maximum selectivity, which was one of the reasons for its lowering. In view of the above, the possibility of irradiating particles in a much wider region of space without appreciable loss in the optical excitation selectivity of clustered molecules is an advantage of the isotope-selective dissociation process. At the same time, the possibility of broadening the absorption spectrum due to the presence of clusters of different sizes and, thereby, a possible decrease in the selectivity should be taken into account here.
In conclusion, we should emphasize that the results that we presented above demonstrate only the potential capability of using the considered processes for the implementation of low-energy technology of laser isotope separation. In fact, they make it possible to estimate the parameters of the elementary separation event such as its selectivity and efficiency/productivity. To develop the technology, it is necessary to solve a whole number of problems. In particular, it is necessary to ensure the possibility of efficient use of the “expensive” laser radiation. This problem can be solved, amongst other things, by the correct choice of the nozzle geometry (profile, slit construction, etc.). Another problem is related to the need for physical separation of the target isotope from the main particle flow. Here, there are also a number of opportunities to realize such a separation [34]. All this allows us to expect that, with sufficient effort, there is a real possibility of developing a new technology for laser separation based on the studied processes or their combinations.
REFERENCES
G. N. Makarov, Phys. Usp. 58, 670 (2015).
J. W. Eerkens, Nucl. Sci. Eng. 150, 1 (2005).
J. W. Eerkens, Laser Part. Beams 23, 225 (2005).
G. N. Makarov and A. N. Petin, J. Exp. Theor. Phys. 103, 697 (2006).
G. N. Makarov, Phys. Usp. 49, 1131 (2006).
J. Kim, J. W. Eerkens, and W. H. Miller, Nucl. Sci. Eng. 156, 219 (2007).
J. Kim et al., in Proceedings of the Spring Meeting, Transactions of the Korean Nuclear Society, Jeju, Korea, 2009.
J. W. Eerkens and J. Kim, AIChE J. 56, 2331 (2010).
G. N. Makarov and A. N. Petin, JETP Lett. 93, 109 (2011).
K. A. Lyakhov and H. J. Lee, Appl. Phys. B 111, 261 (2013).
G. N. Makarov and A. N. Petin, J. Exp. Theor. Phys. 119, 398 (2014).
K. A. Lyakhov and H. J. Lee, J. Laser Appl. 27, 022008 (2015).
K. A. Lyakhov, H. J. Lee, and A. N. Pechen, Sep. Purif. Technol. 176, 402 (2017).
V. N. Bagratashvili et al., Multiple Photon Infrared Laser Photophysics and Photochemistry (Harwood Acad., Chur, 1985).
Multiple-Photon Excitation and Dissociation of Polyatomic Molecules, Vol. 35 of Topics in Current Physics, Ed. by C. D. Cantrell (Springer, Berlin, 1986).
J. L. Lyman, Laser Spectroscopy and its Applications, Vol. 11 of Optical Engineering, Ed. by L. J. Radziemski, R. W. Solarz, and J. A. Raisner (Marcel Dekker, New York, 1987), p. 417.
G. N. Makarov, Phys. Usp. 48, 37 (2005).
V. Yu. Baranov et al., in Proceedings of the 2nd All-Russia Conference on Physicochemical Processes during Selection of Atoms and Molecules, Ed. by V. Yu. Baranov and Yu. A. Kolesnikov (TsNIIatominform, Moscow, 1997), p. 21.
V. S. Letokhov and E. A. Ryabov, in Isotopes: Properties, Production, Application, Ed. by V. Yu. Baranov (IzdAT, Moscow, 2000), p. 329 [in Russian].
V. Yu. Baranov and A. P. Dyad’kin, in Isotopes: Properties, Production, Application, Ed. by V. Yu. Baranov (I-zdAT, Moscow, 2000), p. 343 [in Russian].
V. S. Letokhov and E. A. Ryabov, The Optics Encyclopedia. Basic Faundations and Practical Applications, Ed. by Th. G. Brown, K. Kreath, H. Kogelnik, M. A. Kriss, J. Schmith, and M. J. Weber (Wiley-VCH, Weinheim, 2004), Vol. 2, p. 1015.
J.-M. Zellweger, J. M. Philippoz, P. Melinon, R. Monot, and H. van den Bergh, Phys. Rev. Lett. 52, 522 (1984).
V. M. Apatin, G. N. Makarov, N.-D. Ogurok, A. N. Petin, and E. A. Ryabov, J. Exp. Theor. Phys. 127, 244 (2018).
G. N. Makarov, N.-D. Ogurok, and A. N. Petin, Quantum Electron. 48, 667 (2018).
V. M. Apatin, V. N. Lokhman, G. N. Makarov, N.‑D. Ogurok, and E. A. Ryabov, J. Exp. Theor. Phys. 125, 531 (2017).
V. M. Apatin, V. N. Lokhman, G. N. Makarov, N.‑D. Ogurok, and E. A. Ryabov, Quantum Electron. 48, 157 (2018).
R. S. McDowell, B. J. Krohn, H. Flicker, and M. C. Vasquez, Spectrochim. Acta, A 42, 351 (1986).
J. Geraedts, S. Setiadi, S. Stolte, and J. Reuss, Chem. Phys. Lett. 78, 277 (1981).
J. Geraedts, S. Stolte, and J. Reuss, Z. Phys. A 304, 167 (1982).
J. Geraedts, M. Waayer, S. Stolte, and J. Reuss, Faraday Discuss. Chem. Soc. 73, 375 (1982).
P. Melinon, R. Monot, J.-M. Zellweger, and H. Bergh, Chem. Phys. 84, 345 (1984).
G. Baldacchini, S. Marchetti, and V. Montelatici, J. Mol. Spectrosc. 91, 80 (1982).
R. J. Jensen, J. G. Marinuzzi, C. P. Robinson, and S. D. Rockwood, Laser Focus 12, 51 (1976).
J. M. Philippoz, B. Calpini, R. Monot, and H. van den Bergh, Ber. Bunsen-Ges. Phys. Chem. 89, 281 (1985).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Apatin, V.M., Lokhman, V.N., Makarov, G.N. et al. Isotope Selective Control over Clustering of SF6 Molecules and Dissociation of (SF6)mArn van der Waals Clusters Using an IR Laser. Opt. Spectrosc. 127, 61–68 (2019). https://doi.org/10.1134/S0030400X19070026
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0030400X19070026