Abstract
Endolichenic fungi are considered a promising source of new materials. For further evaluation of some biological activities of the Trichoderma strains isolated from lichens Dirinaria spp. and Cryptothecia spp., their antifungal and antibacterial activities were screened by the methods of dual culture and environmental toxicity. Substrate degradation was evaluated using the qualitative enzyme assays. Fourteen strains of Trichoderma spp. were isolated from 60 lichen samples. All the isolates were able to inhibit the radial growth of tested fungal strains (Bipolaris spp., Colletotrichum spp., Corynespora cassiicola, and Fusarium spp.). Otherwise, only 12/14 isolates were found capable of competing for substrates with Ralstonia solanacearum. The cell-free supernatant obtained from the cultures possessed both antifungal and antibacterial activities. The antagonistic activity of the isolates was selective. Most of the isolates were able to degrade at least one of the investigated substrates, namely cellulose, pectin, and starch. All strains could produce peroxidase; none of the isolates possessed laccase and tyrosinase. A potential antagonistic fungal strain VDT6 has been identified as Trichoderma harzianum. The assessment results indicated that the Trichoderma isolates could be used in agriculture as biological control agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lichens are symbiotic organisms that can survive in a variety of environmental conditions. The diversity of lichen-associated fungi accounts for more than 20% of global fungal biodiversity (Karthikaidevi et al., 2009). The microscopic fungi able to thrive inside the lichen thalli without causing any visible disease symptoms are called endolichenic fungi (ELF). ELF are a new research material with many unique biological activities, such as antibacterial, antifungal, antioxidant, and cytotoxic properties (Kellogg and Raja, 2016; Suryanarayanan and Thirunavukkarasu, 2017). The first endolichenic fungi were isolated and reported by Petrini et al. (1990) when conducting fungal isolation from the fruticose lichens Cladonia and Stereocaulon collected from a forest mountains range in Germany (Petrini et al., 1990). Trichoderma strains have also been isolated as ELF from various lichens, including Parmelia taractica (Girlanda et al., 2008), Parmelia spp. (Padhi and Tayung, 2015), Bulbothrix setschwanensis, Flavoparmelia caperata, Flavoparmelia flaventior, Heterodermia flabellate, Parmotrema reticulatum, Parmotrema thomsonii, Parmotrema crinitum, Physcia dilatata, and Usnea spp. (Tripathi and Joshi, 2019). Most recently, Yang et al. (2021) reported that Trichoderma accounted for 10.77% of endolichenic fungal strains isolated from the lichen Parmotrema tinctorum collected on pine tree trunks in the subtropical area of Jeju Island, Korea. Trichoderma spp. were generally described as effective biological control agents (BCAs) against several plant pathogens (Verma et al., 2007). However, reports on the biological activity of endolichenic Trichoderma spp. are still limited. In this work, we investigated the biological activity of Trichoderma fungi isolated from lichens of the genera Dirinaria and Cryptothecia collected in the Mekong Delta, Vietnam.
MATERIALS AND METHODS
Indicator microorganisms. Indicator microorganisms for antagonistic activity testing, i.e., Bipolaris sp. strain RCT, Colletotrichum spp. strain TCT, Corynespora cassiicola strain CCT, Fusarium sp. strain SCT, and Ralstonia solanacearum strain CBL, were provided by the Plant Gene Technology Laboratory, Biotechnology Research and Development Institute, Can Tho University, Can Tho City, Vietnam.
Isolation of Trichoderma spp. from lichen samples. Samples of Cryptothecia spp. and Dirinaria spp. were collected in the Mekong Delta, Vietnam, including 12 provinces: An Giang (VAG), Bac Lieu (VBL), Ben Tre (VBT), Ca Mau (VCM), Dong Thap (VDT), Hau Giang (VHG), Kien Giang (VKG), Long An (VLA), Soc Trang (VST), Tien Giang (VTG), Tra Vinh (VTV), and Vinh Long (VVL) and the city of Can Tho (VCT). Bark samples with lichens growing on the surface were collected into paper bags and rapidly transported to the laboratory. Identification at the genus level was performed using the taxonomic key described by Elix (2009a, 2009b). The lichen samples were washed under running tap water for 5 min. Then, the lichen thalli were separated from tree bark with a sterile surgical blade under a stereo microscope (ZEISS, Germany). The surface of the thalli was sterilized as follows: 95% ethanol for 30 s, 0.5% NaClO for 2 min, 70% ethanol for 30 s, and rinsed three times with sterile distilled water (Oh et al., 2020). The thalli were dried on sterilized paper and cut into 1 cm2 pieces using sterile surgical blades (Oh et al., 2020). The lichen fragments were placed on potato dextrose agar (PDA) plates (five fragments per plate), and incubated at room temperature for 2 weeks. The names of the endolichenic fungal isolates were coded corresponding to the location where the lichen samples were collected. The isolates were identified as Trichoderma spp., based on the description by Chaverri and Samuels (2003).
Antifungal activities. The Trichoderma isolates were screened for antifungal activities using the dual culture method described by Hamzah et al. (2018) with minor changes. Agar discs (6 mm diameter) of the fungal pathogens and the test Trichoderma isolates were obtained from the growing margin of the colonies. Prepared potato dextrose agar (PDA) plates were divided into two halves. On each plate, a pathogenic fungal disc was placed 1.5 cm from the margin on one half. On the other half, a disc of the tested Trichoderma spp. was placed similarly but directly opposite the pathogen. The plates inoculated with pathogens without antagonistic fungi were used as controls. After seven days, the radial growth of the test pathogen mycelia was measured on the control plate (r1) and the plate with the antagonistic Trichoderma isolate (r2). The experiment was repeated three times. The growth inhibition percentage (GI%) was calculated by the equation: GI% = [(r1– r2)/r1] × 100.
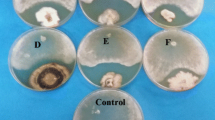
The zone of inhibition (if any) was recorded and the interactions between the indicator strains and the isolates were evaluated according to the following criteria (Mejía et al., 2008; Hamzah et al., 2018): (1) mutual intermingling growth: both fungi grow in separate sections without any signs of macroscopic interaction (Fig. 1a); (2) antibiosis: growth inhibition is determined by the presence of an inhibitory zone (Fig. 1b); (3) competition for substrate: overgrowth of one organism over another (Fig. 1c); and (4) mycoparasitism: direct parasites on the mycelia of pathogens (Fig. 1d).
Antibacterial activities assay. A suspension of R. solanacearum was prepared in a sterile physiological saline solution with turbidity equal to 0.5 McFarland. Fifty µL of the bacterial suspension was spread evenly onto the Mueller-Hinton agar plate. Fungal discs (6 mm in diameter) from one-week-old Trichoderma plates were placed in the center of the R. solanacearum lawn. Plates inoculated with R. solanacearum alone were used as controls. The test plates were incubated at 2–8°C for 14–18 h for the diffusion of the active substances from the fungal disc into the medium plate. Then, the test plates were incubated at 30°C, and antagonism was observed after 48-h incubation (Nedialkova and Naidenova, 2005). A zone of inhibition (if any) was noted, and the interactions between Trichoderma isolates and R. solanacearum were evaluated according to the criteria described above (Mejía et al., 2008; Hamzah et al., 2018). The experiment was repeated in triplicate.
Antibacterial and Antifungal Activities of Non-Volatile Compounds from the Trichoderma Isolates
Preparation of the cell-free supernatant. Three fungal discs (6 mm) were inoculated into a 250-mL conical flask containing 100 mL of potato dextrose broth (PDB) and incubated at room temperature for 15 days on a shaker at 120 rpm. The culture aliquot was filtered through no. 1 Whatman filter paper under sterile conditions.
Investigation of the ability to inhibit indicator fungi. The cell-free supernatant (CFS) obtained from the Trichoderma isolates was mixed with molten PDA to a final concentration of 20% (vol/vol) and distributed into petri dishes (Hamzah et al., 2018). After solidification, a fungal disc (6 mm in diameter) of the tested pathogen was placed in the center of the freshly prepared medium plates. The pathogenic fungal strains were cultured on a PDA medium as controls. After 7 days, the radial growth of the tested pathogens on a PDA supplemented with CFS (d2) and on the control plates (d1) was measured. The percentage inhibition of mycelia growth was calculated using the formula: GI% = (1 − d2/d1) × 100.
Investigation of the ability to inhibit Ralstonia solanacearum. The cell-free supernatant obtained from the Trichoderma isolates was mixed thoroughly with Mueller-Hinton broth (MHB) to achieve the final concentration of 20% (vol/vol). R. solanacearum suspension prepared as described above (50 µL) was added to 5 mL of the MHB with the CFS (treatment). For control, 5 mL of MHB was used, followed by inoculation of 50 µL of R. solanacearum suspension. After 18 h of incubation, 1 mL of bacterial culture was centrifuged at 13 000 g for 5 min. The pellet was resuspended in 1 mL of sterile physiological saline. The optical density (OD600) of the bacterial suspension was measured using a GENESYS 10 UV Scanning UV/Visible spectrophotometer (Thermo Fisher Scientific, United States). The experiment was repeated three times. Growth inhibition of indicator bacteria of the CFS was determined by the formula: GI% = (1 − OD600 treatment/OD600 control) × 100.
Cultivation on Polymeric Substances
A fungal disc with a diameter of 6 mm was placed in the center of a petri dish containing the medium used for investigation and incubated at room temperature. The experiment was repeated in triplicate.
Starch. The nutrient agar with 5% starch was used to investigate the amylolytic activity of the Trichoderma spp. After 48 h of incubation, the test plates were flooded with 1% Lugol solution, and the clear zone size was recorded (Kannangara et al., 2009).
Cellulose. Cellulose degradation activity of the Trichoderma isolates was investigated on carboxymethyl cellulose (CMC) medium (Eggins and Pugh, 1962) after 3-day incubation at room temperature. Cellulose utilization was assessed by clear zones around the fungal colonies after staining with 1% Lugol solution.
Pectin. The basal medium described by Eggins and Pugh (1962) and supplemented with 0.5% pectin from citrus peel was used. After 2 days of incubation, the plates were coated with 1% cetyltrimethylammonium bromide solution, and clear zones around the fungal colonies were measured (Kannangara et al., 2009).
Lignin. The cultures were inoculated on a PDA medium and incubated for 48 h. The presence of the enzyme was quantified with the corresponding reagent as described by Stalpers (1978) with a minor change. The reaction with α-naphthol (on laccase) revealed a purplish discoloration. A yellowish-brown color occurred in the case of the reactions with pyrocatechol and H2O2 (on peroxidase). The color turns orange-brown when tyrosinase reacts with p-crezol.
Identification of the selected strain VDT6 using ITS sequence analysis. Based on the assessment results, the DNA of strain VDT6 was isolated by the method described by Al-Samarrai and Schmid (2000). Amplification of the internal transcribed spacer (ITS) region was done by the set of primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (Fujita et al., 2001). PCR was carried out in a reaction mixture containing 15.75 µL double distilled water, 5 µL 5× MyTaq DNA polymerase buffer (Bioline, United States), 1 µL 10 μM forward and reverse primers, 1.25 U MyTaq DNA polymerase (Bioline, United States), and 1 µL genomic DNA. PCR was carried out in ABI 9800 Fast Thermal Cycler, with the following conditions: 94°C for 3 min, 35 cycles of 94°C for 30 s, 55°C for 30 s, elongation at 72°C for 1 min, and the final elongation step at 72°C for 7 min. The amplified product was sent for sequencing (Apical Scientific, Malaysia). The obtained sequences (GenBank deposition number: OP108343) were used for homology search against the NCBI database by BLASTn.
Statistical analysis. One-way ANOVA was used to analyze the antagonistic activities of the Trichoderma strains, using MINITAB 16 statistical packages (Minitab, Inc.).
RESULTS
Isolation and identification of the Trichoderma spp. A total number of 71 fungi strains were isolated from 60 samples of Cryptothecia and Dirinaria. From each lichen sample, we isolated only one species of associated fungi, but in some cases, there were more than one fungal genera. The identification results showed a high diversity of endolichenic fungi. The isolated fungal strains belonged to many genera, including Aspergillus, Chaetomium, Calonectria, Fusarium, Penicillium, Trichoderma, Xylaria, etc.
Therein, 14 strains were recorded with the characteristics of the genus Trichoderma. Among these, 12/14 strains were isolated from Dirinaria spp., and 2/14 strains were isolated from Cryptothecia spp. The isolates were fast-growing. Most isolates had mycelia spreading over the PDA plate after 2–3 days. The conidia of the isolates were spherical and transversely truncated at the base, ovoid or ellipsoid. Green ascospores appeared from 2 to 12 days of cultivation on PDA. The mycelium was septate and branched. The conidiophores were symmetrical or alternate. The phialides grew in clusters of 2–5.
Antifungal activities. All of the Trichoderma isolates inhibited the mycelial radial growth of the indicator fungi. The results demonstrated that strains VST2, VDT6, and VDT3 exhibited the highest percentage of inhibition against Bipolaris spp. and Fusarium spp. (Figs. 2a, 2d), Colletotrichum spp. (Fig. 2b), and Corynespora cassiicola (Fig. 2c), respectively. All the cultures exhibited the mycoparasitic interaction with the mycelia of Bipolaris spp., Colletotrichum spp., and Fusarium spp. (Figs. 3a, 3b, 3d, respectively). However, after 7 days of inoculation, only 6 out of 14 strains (VCT1, VCT3, VHG4, VKG5, VLA3, and VST2) exhibited mycoparasitism against Corynespora cassiicola. The rest showed the competition for substrate interaction (Fig. 3c). Observation under the microscope showed that the mycelium of Trichoderma coiled around the mycelium of the tested fungal strains (Figs. 3e, 3f). In general, four strains (VDT6, VST2, and VVL1) showed the most effective antagonistic activity against four tested fungi.
Inhibition of the growth of tested fungi by the Trichoderma isolates: VST2 strain against Bipolaris spp. (a); VDT6 strain against Colletotrichum spp. (b); VDT3 against Corynespora cassiicola (c); VST2 strain against Fusarium spp. (d); mycelium of VDT6 strain (black arrow) coiled around Colletotrichum spp. mycelium (red arrow), light microscopy at 1000× (e); and mycelium of VST2 strain (black arrow) coiled around Bipolaris spp. mycelium (red arrow). Light microscopy at 1000×.
Antibacterial activities. The ability to grow and spread on the surface of the R. solanacearum lawn suggested that 12/14 strains of Trichoderma exhibited competition for substrates and space with R. solanacearum. Moreover, metabolites of Trichoderma strains present in the agar discs and produced from the growing hyphae, could inhibit the growth of R. solanacearum. Strain VDT6 possessed the highest antibacterial activity, followed by VST2 and VHG2 with GI of 39.3, 31.7, and 30.7%, respectively (P < 0.05) (Fig. 2e). The other two strains (VCT1 and VLA3) did not exhibit any antagonistic activities against R. solanacearum. The strong growth of bacteria around the fungal disc was observed to prevent the fungal growth. Strains VDT6, VHG2, and VST2 showed a pronounced inhibitory effect against both R. solanacearum (Fig. 2e) and the tested fungi (Figs. 2a−2d). Four other strains (VDT1, VKG5, VVL1, and VVL4) showed weak inhibitory activity against R. solanacearum (Fig. 1e), but strong inhibition of mycelial growth of the tested fungi (Figs. 2a−2d). Overall, the antagonistic activities of the isolates against test cultures of fungi and bacteria were variable.
Antagonistic activities of non-volatile compounds from the Trichoderma isolates. Assessment results showed the cell-free supernatants (CFS) obtained from 14 fungal strains were able to inhibit the growth of all the tested fungal strains. The CFS obtained from strains VAG1; VHG2 and VLA3; VDT3 and VVL4; VST2 and VVL4 exhibited the most pronounced radial growth inhibition of Bipolaris spp. (Fig. 4a), Colletotrichum spp. (Fig. 4b), Corynespora cassiicola (Fig. 4c), and Fusarium spp. (Fig. 4d), respectively (p < 0.05). The CFS of 12 Trichoderma strains also exhibited the activities against R. solanacearum. The CFS obtained from two strains (VAG1 and VHG4) showed the highest ability to inhibit R. solanacearum (p < 0.05) (Fig. 4e). In comparison with dual culture test results, CFS obtained from the isolates displayed a low inhibitory effect on mycelium growth of the tested fungi. In contrast, the growth inhibition percentage by CFS was recorded at a high rate in the case of R. solanacearum. The CFS activities also depended on the tested microorganism, e.g., strain VAG1 showed the highest activity against Bipolaris spp. (GI% = 29.0%) and R. solanacearum (GI% = 61.0%), but the activity was lower with the other test organisms (GI% was 21.7, 22.3, and 13.7% in the case of Colletotrichum spp., Corynespora cassiicola, and Fusarium spp., respectively). In contrast, strain VVL4 displayed the lowest GI% in the case of Bipolaris spp. (9.7%) and R. solanacearum (21.1%), whereas this strain showed the highest rate of GI% in the case of Corynespora cassiicola (43.3%) and Fusarium spp. (31.3%).
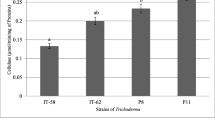
Substrate utilization. As shown in Table 1, strains VAG1, VCT1, VHG4, and VKG5 were unable to degrade starch, cellulose, and pectin. In contrast, three strains (VDT6, VLA3, and VST2) utilized all the tested substrates. The rest were capable of utilizing two out of three tested substrates. The ability to degrade lignin was assessed by producing three enzymes, laccase, peroxidase, and tyrosinase. All strains were able to produce peroxidase, as shown by the yellow-brown diffusion zone. None of the isolates were able to produce tyrosinase and laccase.
Identification of the selected Trichoderma isolate VDT6. R. solanacearum is reported to rank second among the most important phytopathogenic bacteria (Yuliar et al., 2015). In another study, R. solanacearum was found to be the pathogen highly resistant to the metabolites from endolichenic fungi (Jipathi and Joshi, 2019). In this research, the assessment results showed that the strain VDT6 showed the strongest antagonism against R. solanacearum in the dual culture assay (P < 0.05) (Fig. 2e). At the same time, this fungal strain also showed high growth inhibition against the indicator fungi (Figs. 2a−2d). In the case of Colletotrichum spp. and Fusarium spp., strain VDT6 exhibited antifungal activity with no statistically significant difference from strain VST2 (p < 0.05) (Figs. 2b, 2d). CFS obtained from strain VDT6 also showed antibacterial and antifungal activity (Fig. 3). However, spore-formers are the best choice to overcome the obstacles involved in the preservation of the organisms intended as BCA (Yuliar et al., 2015). On the other hand, strain VDT6 showed a strong ability to metabolize macromolecular substrates (Table 1), indicating its potential in the production of BCA biomass from agricultural by-products. BLASTn results indicated that strain VDT6 was similar to Trichoderma harzianum (accession no. ON705517.1) (99.2% identity), T. lixii (accession no. MZ489571.1) (99.3% identity), T. simmonsii (accession no. MK346240.1) (99.2% identity), and T. tawa (accession no. MK346243.1) (99.3% identity). Morphological characteristics of strain VDT6 were also documented. The colonies were fast-growing, and the hyphae were cottony and radiating when grown on PDA. Conidia formed within 24–48 h in broad concentric rings. The color of conidia changed from grayish green to dark green during senescence. No diffusing pigments were recorded. Conidiophores were pyramidal with opposing branches. The main axis and each branch terminated in a whorl of 3–5 phialides (size 7.0–12.0 × 3.0 µm), and the phialides at the top were larger. Conidia were subglobose, truncated at the base to ovoid, smooth, 3.0–6.0 × 2.0–3.0 µm in size. In combination with BLASTn results and morphological characteristics, strain VDT6 was identified as Trichoderma harzianum, as described by Chaverri and Samuels (2003).
DISCUSSION
The Mekong River Delta is located in the tropical sub-equatorial monsoon region. The climate is generally hot and humid (Hong et al., 2021), suitable for the growth of various lichen species. Cryptothecia spp. and Dirinaria spp. were found mainly on tree bark at the collection sites, similar to the description of Elix (2009a, 2009b). In this study, ELF communities from two lichen genera, Cryptothecia and Dirinaria, showed a high degree of diversity. Trichoderma spp. was widespread, accounting for 19.7% of the total ELF isolates. To the knowledge of the research team, this is one of the first studies on endolichenic fungi and endolichenic Trichoderma isolates from Cryptothecia spp. and Dirinaria spp. reported in Vietnam.
The dual culture method revealed high antagonistic activity of the Trichoderma strains, which exhibited activity against the tested pathogenic fungi and R. solanacearum. Moreover, mycoparasitic interaction and pigment production have also been observed in the co-culture plates containing the pathogenic fungi and some of the Trichoderma isolates. Rapid metabolism, production of inhibitory metabolites, and physiological conformation (mycelia, conidia, and chlamydospores) have been reported to be the major contributors to the antagonistic potential of Trichoderma spp. (Verma et al., 2007). The production of microbial inhibitory metabolites can help the isolates to grow over the colonies of the tested fungi and R. solanacearum. Parasitism can cause nutrient depletion and thus lead to the breakdown of pathogens. Pigment production was documented as the defense mechanism of the fungi to protect them from other microbes (Hamzah et al., 2018). All the tested fungal pathogens showed strong pigment production in the dual culture plates with Trichoderma. However, many Trichoderma isolates still exhibited parasitic activity on the colonies of pathogenic fungi. In general, the antagonistic effect of the dual culture method was achieved by the combination of microbial inhibitory factors and the competition between the antagonistic fungi and the indicator microorganisms.
In contrast, the antibacterial and antifungal activities of CFS obtained from Trichoderma strains depended on the indicator microorganism. Biologically active compounds from Trichoderma spp. have been revealed as secondary metabolites, hydrolytic enzymes (chitinase, protease, and β-glucanases) (Verma et al., 2007; Li et al., 2016), or peptaibols (Li et al., 2016). The inhibitory activity of the metabolites depended on the sensitivity of the microorganisms examined. The detoxification capacity of the pathogens may protect them from the metabolites (Hamzah et al., 2018).
Yield losses in agriculture caused by plant pathogens are estimated at 16% (Ficke et al., 2018). Biological control agents (BCAs) can control plant pathogens via competitive microbial interactions and stimulation of resistance systems in plants. BCAs also have the advantages of being environmentally friendly, self-sustaining, and reducing costs for non-renewable energy sources (Yuliar et al., 2015). In this study, the Trichoderma isolates showed both antifungal and antibacterial activities against the tested plant pathogens. On the other hand, the ability to produce enzymes to degrade various substrates can help the antagonists to compete for spaces with pathogens. Moreover, macromolecular substrates such as starch, cellulose, pectin, and lignin compound are agricultural by-products. The potential to utilize agricultural by-products for the production of commercial Trichoderma spp. biomass products will bring high value to agricultural cultivation.
REFERENCES
Al-Samarrai, T.H. and Schmid, J., A simple method for extraction of fungal genomic DNA, Lett. Appl. Microbiol., 2000, vol. 30, pp. 53–56. https://doi.org/10.1046/j.1472-765x.2000.00664.x
Chaverri, P. and Samuels, G.J., Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with Green Ascospores, Utrecht: Centralbureau voor Schimmelcultures, 2003.
Eggins, H.O.W. and Pugh, G.J.F., Isolation of cellulose-decomposing fungi from the soil, Nature, 1962, vol. 193, pp. 94–95. https://doi.org/10.1038/193094a0
Elix, J.A., Cryptothecia, in Flora of Australia. Volume 57: Lichens 5, McCarthy, P.M. and Kuchlmayr, B., Eds., Victoria: CSIRO/ABRS, 2009a, pp. 1–12.
Elix, J.A., Dirinaria, in Flora of Australia. Volume 57: Lichens 5, McCarthy, P.M. and Kuchlmayr, B., Eds., Victoria: CSIRO Publishing/ABRS, 2009b, pp. 509–517.
Ficke, A., Cowger, C., Bergstrom, G., and Brodal, G., Understanding yield loss and pathogen biology to improve disease management: Septoria nodorum blotch—a case study in wheat, Plant Dis., 2018, vol. 102, pp. 696–707. https://doi.org/10.1094/PDIS-09-17-1375-FE
Fujita, S.I., Senda, Y., Nakaguchi, S., and Hashimoto, T., Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains, J. Clin. Microbiol., 2001, vol. 39, pp. 3617–3622. https://doi.org/10.1128/JCM.39.10.3617-3622.2001
Girlanda, M., Isocrono, D., Bianco, C., and Luppi-Mosca, A.M., Two foliose lichens as microfungal ecological niches, Mycologia, 2008, vol. 89, pp. 531–536. https://doi.org/10.1080/00275514.1997.12026814
Hamzah, T.N.T., Lee, S.Y., Hidayat, A., Terhem, R., Faridah-Hanum, I., and Mohamed, R., Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani, Front. Microbiol., 2018, vol. 9, p. 1707. https://doi.org/10.3389/fmicb.2018.01707
Hong, N.V., Hien, N.T., Minh, N.T.T., and Toan, H.C., Forecasting saline intrusion under the influence of the northeast monsoon in the Mekong Delta, VN J. Hydrometeorol., 2021, vol. 9, pp. 23–36. https://doi.org/10.36335/VNJHM.2021(9).23-36
Kannangara, B.T., Rajapaksha, R.S., and Paranagama, P.A., Nature and bioactivities of endolichenic fungi in Pseudocyphellaria sp., Parmotrema sp. and Usnea sp. at Hakgala montane forest in Sri Lanka, Lett. Appl. Microbiol., 2009, vol. 48, pp. 203–209. https://doi.org/10.1111/j.1472-765X.2008.02512.x
Karthikaidevi, G., Thirumaran, G., Manivannan, K., Anantharaman, P., Kathiresan, K., and Balasubaramanian, T., Screening of the antibacterial properties of lichen Roccella belangeriana (Awasthi) from Pichavaram mangrove (Rhizophora sp.), Adv. Biol. Res., 2009, vol. 3, pp. 127–131.
Kellogg, J.J. and Raja, H.A., Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon, Phytochem. Rev., 2016, vol. 16, pp. 271–293. https://doi.org/10.1007/s11101-016-9473-1
Li, Y., Sun, R., Yu, J., Saravanakumar, K., and Chen, J., Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum, Indian J. Microbiol., 2016, vol. 56, pp. 318–327. https://doi.org/10.1007/s12088-016-0581-9
Mejía, L.C., Rojas, E.I., Maynard, Z., Bael, S.V., Arnold, A.E., Hebbar, P., Samuels, G.J., Robbins, N., and Herre, E.A., Endophytic fungi as biocontrol agents of Theobroma cacao pathogens, Biol. Control, 2008, vol. 46, pp. 4–14. https://doi.org/10.1016/j.biocontrol.2008.01.012
Nedialkova, D. and Naidenova, M., Screening the antimicrobial activity of actinomycetes strains isolated from Antarctica, J. Cult. Collect., 2005, vol. 4, pp. 29–35.
Oh, S.Y., Yang, J.H., Woo, J.J., Oh, S.O., and Hur, J.S., Diversity and distribution patterns of endolichenic fungi in Jeju Island, South Korea, Sustainability, 2020, vol. 12, p. 3769. https://doi.org/10.3390/su12093769
Padhi, S. and Tayung, K., In vitro antimicrobial potentials of endolichenic fungi isolated from thalli of Parmelia lichen against some human pathogens, Beni-Suef Univ. J. Basic Appl. Sci., 2015, vol. 4, pp. 299–306. https://doi.org/10.1016/j.bjbas.2015.11.006
Petrini, O., Hake, U., and Dreyfuss, M.M., An analysis of fungal communities isolated from fruticose lichens, Mycologia, 1990, vol. 82, pp. 444–451. https://doi.org/10.1080/00275514.1990.12025907
Stalpers, J.A., Identification of Wood-Inhabiting Fungi in Pure Culture, Baarn: Centraalbureau voor Schimmelcultures, 1978.
Suryanarayanan, T.S. and Thirunavukkarasu, N., Endolichenic fungi: the lesser known fungal associates of lichens, Mycology, 2017, vol. 8, pp. 189–196. https://doi.org/10.1080/21501203.2017.1352048
Tripathi, M. and Joshi, Y., Endolichenic Fungi: Present and Future Trends, Singapore: Springer, 2019.
Verma, M., Brar, S.K., Tyagi, R.D., Surampalli, R.Y., and Valéro, J.R., Antagonistic fungi, Trichoderma spp.: panoply of biological control, Biochem. Eng. J., 2007, vol. 37, pp. 1–20. https://doi.org/10.1016/j.bej.2007.05.012
Wheeler, K.A. and Hocking, A.D., Interactions among xerophilic fungi associated with dried salted fish, J. Appl. Bacteriol., 1993, vol. 74, pp. 164–169. https://doi.org/10.1111/j.1365-2672.1993.tb03010.x
Yang, J.H., Oh, S.Y., Kim, W., Woo, J.J., Kim, H., and Hur, J.S., Effect of isolation conditions on diversity of endolichenic fungal communities from a foliose lichen, Parmotrema tinctorum, J. Fungi (Basel), 2021, vol. 7, p. 335. https://doi.org/10.3390/jof7050335
Yuliar, Nion, Y.A., and Toyota, K., Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum, Microbes Environ., 2015, vol. 30, pp. 1–11. https://doi.org/10.1264/jsme2.ME14144
ACKNOWLEDGMENTS
N.T. Phu was funded by Vingroup Joint Stock Company and supported by the Domestic Master/Ph.D. Scholarship Programme of Vingroup Innovation Foundation, Institute of Big Data (VNCDLL), code VINIF.2021.ThS.61. The authors would like to acknowledge the Vingroup Innovation Foundation, Institute of Big Data for the financial support.
Funding
This study was supported by the Vingroup Joint Stock Company and the Domestic Master/Ph.D (Project/grant number VINIF.2021.ThS.61), Vietnam.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Phu, N.T., Cam, V.T., Minh, N.T. et al. Biological Activity of the Endolichenic Trichoderma spp. Isolated from Lichens Cryptothecia spp. and Dirinaria spp.. Microbiology 92, 408–417 (2023). https://doi.org/10.1134/S0026261722602093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602093