Abstract
Plant-soil interactions directing plant growth are governed by chemical communications among the microorganisms, and between the plants and microbes. A study was conducted to evaluate the effects of seed biopriming with native plant-growth promoting rhizobacteria (PGPR) on growth parameters of wheat. Rhizospheric bacteria isolated from drought-exposed fields were characterized on morphological, biochemically and molecular basis and screened for PGP traits. Nine isolates were able to solubilize essential nutrients, produce plant growth hormone indole acetic acid (IAA), and most of the isolates were positive for siderophore, ammonia, hydrogen cyanide (HCN), and hydrolytic enzyme production. Considerable and varying amounts of exopolysaccharides (EPS) constituted of proteins (amide I, II, III), polysaccharides, nucleic acids and peptidoglycan were produced by few strains that helped in formation of biofilm matrix. Biopriming wheat seeds with selected bacterial isolates brought significant increase in wheat germination, growth, and yield parameters. Correlation analysis revealed that the phytohormone, phosphate solubilization and EPS-producing abilities were the most obvious PGP traits of bacteria related to the growth and yield of wheat plants. Among all the nine bacterial isolates tested, two isolates, viz. Pseudomonas azotoformans JRBHU5 and Burkholderia seminalis JRBHU6, with good colonizing abilities enhanced plant vigor at early stage thereby augmenting wheat growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Increasing agricultural production through utilization of natural biota has become even more relevant under the changing climatic conditions. Plant growth-promoting rhizobacteria (PGPR) are an indispensable part of the rhizosphere biota which get established in soil ecosystem due to their high adaptability over a wide range of environmental factors, high colonizing abilities and biochemical versatility, which enables them to metabolize a broad range of natural and xenobiotic compounds. PGPRs stimulate plant growth and offer host protection against biotic and abiotic stresses either through direct mechanisms like nutrient solubilization, nitrogen fixation, production of plant hormones, and lowering ethylene levels (Raghuwanshi and Prasad, 2018) or indirect mechanisms, like siderophore, hydrogen cyanide (HCN) and antibiotic production and synthesis of cell wall-lysing enzymes (Premachandra et al., 2020). Exopolysaccharides (EPS) are high molecular weight polymeric materials of microbial origin which promote microbial aggregation by initiating surface adherence and binding soil particles through Van der Waals force, hydrogen bonds, cation bridges, and anion adsorption mechanism which not only endorse stability of soil aggregates but also increases soil moisture content (Vardharajula, 2021), protecting plant roots from extreme desiccation (Jochum et al., 2019). The complex interactions and dynamics of microorganisms, roots, soil, and water induce changes in the physico-chemical and structural properties of the rhizosphere which impacts a range of processes influencing crop yield like the regulation and diffusion of organic carbon sources (Dubey et al., 2021).
Wheat (Triticum aestivum L.) is extensively cultivated globally over an area of about 239.63452 million ha with annual production of nearly 899.37077 million tonnes (FAOSTAT 2019). Increasing wheat production under shrinking agricultural lands and changing climatic conditions has become a challenge, and in this context search for a potent biofertilizer to enhance wheat production becomes relevant. Therefore, the present study aimed to isolate bacterial communities from roots to compare their growth-promoting abilities and check their influence on wheat growth through seed biopriming.
MATERIALS AND METHODS
Soil sample collection. Soil samples were collected from the rhizospheric regions of pigeon pea (Cajanus cajan L.), wheat (Triticum aestivum L.), and maize (Zea mays L.) plants growing in the states of Uttar Pradesh, Bihar, and Madhya Pradesh (India). The texture of the soil was silty loam with good permeability. Soil samples were collected during April to June (average temperature 35–45°C, average rainfall 525 mm), from a depth of 0–15 cm in “V” shape with the help of a scabbard and stored in sterile poly bags in a cooling box for further study.
Isolation and preliminary screening of bacterial isolates. Bacteria were isolated from collected soil samples by the serial dilution plate technique using the yeast mannitol agar (YEMA) media. Each dilution was spread on petri plates and was incubated at 30°C for 24–48 h. Colonies were picked from these plates and maintained as pure cultures in YEMA media with periodic transfer to fresh media and stock for further use.
Biochemical characterization. All the isolates were characterized based on colony morphology and Gram staining. The methyl red and Voges−Proskauer test were performed to differentiate between members of the family Enterobacteriaceae and also to characterize other groups of bacteria, including Actinobacteria; the citrate test and mannitol test were done to check utilization of the carbon sources by the bacterial isolates, the TSI (triple sugar iron) test was performed to understand the ability of the bacterial isolates to ferment sugars with production of hydrogen sulfide. The catalase test checked the catalase activity in bacteria. All biochemical characterization of the purified selected isolates was done according to the standard procedures (Cappuccino and Sherman, 2008; Kumar et al., 2014).
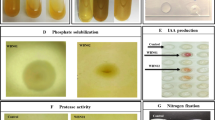
In vitro screening for potential plant growth-promoting traits. A total of 27 bacterial isolates obtained from rhizospheric soil samples were screened for different PGP attributes. IAA production, phosphate solubilization, zinc solubilization, chelation of potassium, siderophore production, HCN production, and ammonia production were measured according to the standard procedures reported elsewhere (Cappuccino and Sherman, 2008; Dey and Raghuwanshi, 2020; Yasmin et al., 2020a). Extracellular enzyme assay was performed to estimate the activities of proteases, ureases, amylases, chitinases, and cellulases (Yasmin et al., 2020a).
Molecular identification. Among all the 27 bacterial isolates, 11 bacterial strains screened for their growth-promoting potential were subjected to molecular identification. The 16S rRNA gene sequence analysis was performed as described previously by Coenye et al. (2001). Bacterial DNA was isolated using a spin column kit (HiPer® Bacterial Genomic DNA Extraction Teaching Kit, Himedia, India). The universal bacterial primers Forward: 530F (5′-GCTCTAGAGCTGACTGACTGAGTGCCAGCMGCCGCGG-3′); Reverse: 800R (5'-TACCAGGGTATCTAATCC-3') were used for the amplification of the 16S rRNA gene sequence. The 16S rRNA gene sequences of the bacterial isolates were submitted to NCBI GenBank under accession numbers listed in Supplementary Table 1.
Phylogenetic analysis based on 16S rRNA. Sequence similarity searches were performed at the National Center for Biotechnology Information (NCBI) server using BLASTN (http://www. ncbi.nlm.nih.gov/blast). The sequences were aligned using Clustal W version 1.8 (Altschul et al., 1997) and subjected to phylogenetic analysis. The phylogenetic tree of the 16S rRNA gene sequences constructed using the Neighbor-Joining method contained 11 bacterial nucleotide sequences. The final data set consisted of 1528 positions. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown next to the branches. The tree was drawn to scale, with branch lengths (next to the branches) in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The phylogenetic distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Evolutionary analyses were conducted in MEGA11 (Tamura et al., 2021).
EPS extraction, purification, and characterization. For EPS extraction, bacterial isolates were grown in the optimized mineral salt medium for 10 days (Bramchari and Dubey, 2006). Aliquots of bacterial cultures (250 mL) were centrifuged at 4°C for 20 min at 15 093 g. Subsequently the supernatant was collected and EPS was extracted by adding double volume of 95% chilled ethanol and for complete precipitation it was kept at 4°C. The extracted EPS were washed with 70% ethanol mixture and dissolved in double-distilled H2O followed by dialysis with dialysis tubing (molecular weight cut-off of 13 kDa; Sigma-Aldrich Chemie GmbH, Seelze, Germany) against double distilled H2O at 4°C for 24 h for removal of excess salts from EPS. Extracted EPS were lyophilized with a CHRIST lyophilizer at 20.68 N/mm2 and stored at room temperature (Bramchari and Dubey, 2006). Lyophilized EPS (10 µg) was suspended in 2 mL of each solvent, viz. benzene, water, chloroform, acetone, ethanol and methanol to check the solubility of EPS. The mixture was vortexed and left undisturbed to observe the pellet formed.
Identification of the matrix components in EPS. The EPS of 9 identified bacterial isolates were subjected to Fourier transform infrared spectroscopy analysis using a JASCO FT/IR-4700 spectrometer to identify the matrix components forming the biofilms. For FTIR analysis, homogeneous mixture of EPS was made by grinding a pinch of EPS sample obtained from a culture with dry solid KBr in a mortar. The mixture was used for making pellets by applying mechanical pressure using a hydraulic press with a pumping movement. The sample hence prepared was further used for FTIR analysis. The measurement was carried out at infrared spectra in the region of 500–4000 nm cm−1, using ATR accessories.
Phenotypic tree construction. Hierarchical clustering routines to produce a dendrogram which can help in understanding the clustering of bacterial isolates on the basis of potential plant growth-promoting traits was done. The dendrogram was constructed using the UPGMA (Unweighted Pair Group Method with Arithmetic Mean) clustering method (Sneath and Sokal, 1973). The similarity association matrix upon which the clusters are based is Bray-Curtis similarity indices. The bootstrap value (1000 replicates) is given at the root of the subtree specifying each group. The analyses were conducted in PAST (Paleontological Statistics) software (version 4.03).
Seed biopriming. Out of the 27 bacterial isolates, nine bacterial isolates with promising plant growth-promoting traits were tested on wheat in the soil system. Seeds of wheat variety BHU-35 were surface-sterilized with 0.2% HgCl2 for 2 min, then rinsed in sterile distilled water for 10 min. Wheat seeds were bioprimed by soaking them in suspensions of pre-screened bacterial isolates in YEM medium (109 CFU mL–1) and was kept at 28 ± 2°C in a rotator shaker (120 rpm) for 7–8 h. The control seeds were soaked in sterile medium. The seeds were then dried overnight aseptically under laminar air flow and used for greenhouse experiments. The seeds were sown in plastic pots containing autoclaved soil and placed in a temperature-controlled growth chamber. Five plants per pot with soil kept at 60% water holding capacity were maintained throughout the experimental period. The effect of PGPR treatment on percent seed germination (PG), seedling shoot length (SL), seedling root length (RL), seedling fresh weight (FW), seedling dry weight (DW), and seedling vigor index (VI) of wheat were determined. Germination and vigor analysis were carried out by the paper towel method (ISTA 1993). The treatments were arranged in a completely randomized design with three replications.
Plant growth and test weight under pot conditions. To study the effect of isolated bacterial strains on growth of wheat var. BHU-35 under pot conditions, experiments were conducted during winter season of 2017–2018 and 2018–2019. Ten bacteria-treated seeds were sown in each pot. Seeds treated with YEM broth without any bacterial strain were used as the control. Pots were arranged in a completely randomized design. Plant growth parameters: plant height (PH), flag leaf area (FLA), numbers of tillers (NT), grain per spike (GPS), days to 50% heading (DH), days to 50% maturity (DM), and thousand grain weight (TGW), were recorded in each replicate as and when required. Biomass was dried to constant weight in an oven at 80°C for recording the dry weight (DW).
Statistical analysis. The experiment was performed in a completely randomized design. For all experiments, data were subjected to statistical analysis using IBM SPSS Statistics v. 25 software package. Data were subjected to analysis of variance (ANOVA) and correlation co-efficient using Duncan’s multiple range test (P ≤ 0.05).
RESULTS AND DISCUSSION
Morphological and biochemical characterization of the isolates. In the present investigation 27 bacterial isolates were screened for their growth-promoting attributes, of which nine isolates performed extremely well. All microbial strains were rod shaped and gram-negative except, Bacillus subtilis JRBHU11. Out of 27 strains, 6 were positive for methyl red, 11 strains were positive for the VP (Voges–Proskauer) test, and 11 strains were positive for fermentation of mannitol. All the isolates were positive for catalase and most of the bacterial isolates showed acidic reaction on citrate agar and triple sugar iron agar (TSI) medium, except Burkholderia paludis JRBHU1, Burkholderia paludis JRBHU2, Pantoea dispersa JRBHU3, Pseudomonas lactis JRBHU4, Pseudomonas azotoformans JRBHU5, Enterobacter hormaechei JRBHU8 and Enterobacter hormaechei JRBHU9 (Supplementary Table 2). Catalase plays a major role in protection against toxic free radicals and is therefore an important parameter in bacterial screening.
In vitro screening of isolates for potential plant growth-promoting traits. As the plant root exudates vary, the rhizospheric microorganisms also show specificity in their association and activity in response to the exudates. Active IAA producers have a direct benefit on plant growth by increasing the root length through cell enlargement/division, growth rate and increased root initiation (Oleńska et al., 2020). High and low levels in IAA production depend upon the genetic variations and carbon utilization rate of the microorganisms. IAA production quantified in the present study ranged from 9.002–149.278 μg/mL, which was much higher than the earlier reports and indicated a broadly diverse bacterial population in the rhizosphere (Prasad et al., 2017) (Table 1). Maximum IAA production was observed by Pa. dispersa JRBHU3 (149.278 µg/mL), followed by Enterobacter cloacae JRBHU10 (65.448 µg/mL) and Ba. subtilis JRBHU11 (49.511 µg/mL).
Among the 27 selected isolates, 26 isolates were good phosphorus solubilizers. Maximum phosphate solubilization index of 46.30 mm was measured around the isolate Burkholderia seminalis JRBHU6, followed by En. hormaechei JRBHU9 (44.40 mm) and Bu. paludis JRBHU1 (42.10 mm). The halo zone formed by the different isolates showed a wide range (8 to 46.30 mm) as shown in Table 1. Good P solubilization is generally due to the production of organic acids (Yasmin et al., 2020b) or due to bacterial synthesis and release of phosphatases, like phosphomonoesterase, phosphodiesterase, and phosphotriesterase, which catalyze the hydrolysis of phosphoric esters. Solubilization of soil phosphorus by microorganisms is highly desirable and eco-friendly, as phosphorus plays a pivotal role in many physiological activities and its deficiency limits crop growth. The solubilization of potassium based on the halo zone formed around the colonies of the isolates spot inoculated on Alekseandrov’s agar medium is shown in Table 1. The highest solubility index was recorded around isolate Bu. seminalis JRBHU6 (46.36 mm), followed by Bu. paludis JRBHU2 (44.66 mm on average) and Bu. paludis JRBHU1 (44.33 mm on average), respectively. Potassium not only plays a key role in developing resistance against biotic and abiotic stress but is fundamentally involved in activating ~80 different enzymes in plants (Etesami et al., 2017).
The bacteria capable of solubilizing zinc in plates produced clear halo around the colony (Table 1). Isolates Bu. paludis JRBHU1 (60 mm) and Bu. paludis JRBHU2 (54.38 mm) showed highest zinc dissolution followed by En. hormaechei JRBHU9 (50.04 mm) and Bu. seminalis JRBHU6 (48.26), among the twenty-three zinc solubilizing bacteria. Bacillus velezensis B26 from Brachypodium distachyon (Sharma et al., 2020) and Pseudomonas sp. and Bacillus sp. from soybean rhizosphere have been reported as good zinc solubilizers. Possible mechanisms for zinc solubilization by microorganisms include the production of organic acids (Alexander, 1998), which results in soil acidification, improving zinc sequestration. Other mechanisms involved in zinc solubilization include the production of siderophores (Saravanan et al., 2011) and chelated ligands (Wakatsuki, 1995).
Siderophore production during rhizosphere colonization has also been recorded as one of the important mechanisms adopted by certain PGPRs (Bradyrhizobium japonicum, Rhizobium leguminosarum, and Sinorhizobium meliloti) to compete for iron acquisition with other microflora present in the soil (Carson et al., 2000). Most of the selected bacterial strains when grown on CAS medium were able to produce siderophores that have an affinity for ferric ions. Maximum halo zone was observed around isolate Bu. seminalis JRBHU6, followed by Bu. paludis JRBHU1 and Ba. subtilis JRBHU11 (Table 1) when grown on CAS plates.
The production of HCN in more than trace amount plays a critical role in the control of fungal pathogens (Flaishman et al., 1996). Among all the 27 isolates, 14 isolates were recorded positive for HCN production as was evident by the change in the color of filter paper from yellow to moderate and reddish brown. In the presence of glycine and FeCl3, a deep brown color of filter paper was observed, giving a clear indication of HCN production by bacterial strains (Table 1).
Microorganisms are also a good source of hydrolytic enzymes as compared to other sources which contribute to nutrient mobilization. Among 27 bacterial isolates, 20 showed amylase activity which was classified into three categories (high, moderate, and weak) based on the amount of starch hydrolyzed (Table 1). While Ba. subtilis JRBHU11 showed the highest starch hydrolysis activity, moderate to good activity was observed in Bu. seminalis JRBHU6. Most of the bacteria were positive for the production of urease, an enzyme playing a vital role in nitrogen supply to plants. Ureases catalyze the hydrolysis of urea to CO2 and NH3, which is a vital process in regulating N supply to plants after fertilizing the fields with urea. Urease activity in soil provides better management of urea fertilizers as it also checks the nitrogen loss due to leaching. The production of lysing enzymes like chitinase, cellulase and protease plays a critical role in antagonistic behavior of microorganism against phytopathogens. Bu. seminalis JRBHU6, En. hormaechei JRBHU9, and En. cloacae JRBHU10 were positive for all chitinase, cellulase and protease activity exhibiting their biocontrol and nutrient-mobilizing potential. Bu. seminalis JRBHU6 has already been reported for its bioactive metabolites pyrrolo(1,2-a)pyrazine-1,4-dione,hexahydro (PPDH) and pyrrolo(1,2-a)pyrazine-1,4-dione,hexahydro-3(2-methylpropyl) (PPDHMP), which showed growth-suppressing activities toward multidrug resistant Staphylococcus aureus and fungal strains, viz. Fusarium oxysporum, Aspergillus niger, Microsporum gypseum, Trichophyton mentagrophytes, and Trichoderma harzianum (Prasad et al., 2021). Chitinase and β-1,3-glucanase are the key enzymes involved in the decomposition of fungal hyphal wall. Elshafie et al. (2012) reported that the key enzymes released by Burkholderia gladioli pv. Agaricicola associated with the dissolution of the cell wall of Fu. oxysporum are chitinase, glucanase, and protease.
Identification of exopolysaccharides. EPS synthesized by bacteria are highly hydrated polymers which are involved in adhesion, water retention, soil aggregation, export, binding of enzymes, exchange of genetic information, and absorption of organic/inorganic compounds (Costa et al., 2018). Moreover, it acts like a protective barrier, source of nutrients, donor or acceptor of electrons, and sink for excess energy (Flemming and Wingender, 2010). Therefore, EPS extracted from the nine isolates in the present study, was quantified to identify the best EPS-producing bacteria. Significant and maximum EPS production was shown by Bu. paludis JRBHU1 (5.85 mg/mL), followed by En. cloacae JRBHU10 (4.39 mg/mL), Ps. azotoformans JRBHU5 (3.96 mg/mL), Bu. seminalis JRBHU6 (3.89 mg/mL) and Pa. dispersa JRBHU3 (3.65 mg/mL). Ps. lactis JRBHU4 (2.69 mg/mL) showed minimum production among the 9 selected bacterial isolates. Sandhya et al. (2009) reported that sunflower seedlings inoculated with EPS-producing Pseudomonas putida GAP-P45 showed improved growth and survival rates under drought-stressed condition by synthesizing a rhizobacterial biofilm matrix around the plant root under water deficiency. EPS gives protection to the roots against desiccation because of its water holding capacity thereby supporting the host plant to tolerate drought stress.
Exopolysaccharide components present in the selected bacterial isolates were further investigated by FTIR spectroscopy. The extracellular exopolysaccharide matrices are highly hydrated polymers that are mainly composed of polysaccharides, proteins, and DNA. Ps. azotoformans JRBHU5 and En. hormaechei JRBHU9 showed intense peaks of amide I (1600–1700 cm–1), amide II (1500–1600 cm–1), and amide III (1240–1350 cm–1) corresponding to a protein-enriched biofilm matrices. These regions showed the presence of –C–O carboxyl groups, –C=O carbonyl stretching and –N–H amide groups of amino acids. The protein components show the characteristic IR band through C=O stretching at the amide I region, in contrast to the C–N bending and the N–H stretching at the amide II region and the amide III region (Mosharaf et al., 2018). The protein content of EPS induces membrane stability and protects the cell by providing osmotic balance in saline conditions. Pa. dispersa JRBHU3 and Bu. seminalis JRBHU6 showed intense peaks near 1220–1250 cm–1 indicating a nucleic acid rich biofilm matrix. The extracellular nucleic acid is a component of biofilm matrix produced by selected bacterial strains which are a good source of genetic exchange, signalling, attachment, and moreover a very important structural component (Costa et al., 2018). Intense peaks observed in the region of 900–1150 cm–1 indicated diverse polysaccharides produced by most of the selected bacterial isolates, the band region of polysaccharides principally resulted from a stretching vibration of C–C and C–O bonds and the deformation of C–O–H and C–O–C bonds (Naumann, 2001) (Fig. 1). Thus, all the components of biofilm matrices, viz. proteins, amides (A&B), polysaccharides, and nucleic acids were found to be present in EPS which aided in water retention, nutrients entrapment, and protection against drought (Wang et al., 2015).
Detection of organic macromolecules present in different exopolysaccharides produced by PGPRs by FTIR analysis. (Where: (1) Ba. subtilis JRBHU11; (2) Bu. seminalis JRBHU6; (3) En. hormaechei JRBHU8; (4) En. hormaechei JRBHU9; (5) Bu. paludis JRBHU1; (6) Pa. dispersa JRBHU3; (7) En. cloacae JRBHU10; (8) Ps. azotoformans JRBHU5; (9) Ps. lactis JRBHU4.)
Phylogenetic analysis based on 16S rRNA gene sequences. As per the phylogenetic tree based on 16S rRNA gene sequences (Fig. 2b), Ba. subtilis JRBHU11 formed an outgroup. The remaining 10 bacterial isolates got classified into two different clusters (1 and 2). Cluster 1 was distributed in two genomic subgroups (1a and 1b). In subgroup 1a, isolates En. cloacae JRBHU10 and En. cloacae JRBHU7 formed a sister monophyletic lineage supported by 93% bootstrapping value. En. hormaechei JRBHU9 and En. hormaechei JRBHU8 showed high relatedness with bootstrap (BT) value 95%. Subgroup 1b comprising of two isolates Ps. azotoformans JRBHU5 and Ps. lactis JRBHU4, formed sister taxa and were highly related (BT: 91%). Cluster 2 comprised of Bu. paludis JRBHU1, Bu. seminalis JRBHU6 and Bu. paludis JRBHU2 which exhibited monophyletic lineage supported by bootstrap value 53%.
Phenotypic tree analysis. To understand the relatedness between the 11 selected bacterial isolates, a dendrogram was constructed based on their in-vitro growth-promoting traits and was compared with the relatedness based on the phylogenetic tree prepared through 16S rRNA sequences. The phenotypic dendrogram analysis classified the 11 bacterial isolates into 2 prominent groups on the basis of plant growth-promoting traits (Fig. 2a). Group 1 comprised of Ps. lactis JRBHU4, Ps. azotoformans JRBHU5, En. hormaechei JRBHU8, En. cloacae JRBHU10 and En. cloacae JRBHU7. Ps. lactis JRBHU4 and Ps. azotoformans JRBHU5 exhibited sister groups which showed high linkage with bootstrap value 93% and high similarity index (0.97). En. hormaechei JRBHU8 showed a similarity index of 0.90 with Ps. lactis JRBHU4 and Ps. azotoformans JRBHU5. Group 2 consisted of Bu. seminalis JRBHU6, Bu. paludis JRBHU2, Bu. paludis JRBHU1 and En. hormaechei JRBHU9. Bu. paludis JRBHU1 and Bu. paludis JRBHU2 formed a sister group with high relatedness (BT: 93%) and a similarity index of 0.97. Bu. seminalis JRBHU6 exhibited a similarity index of 0.89 with Bu. paludis JRBHU1 and Bu. paludis JRBHU2.
Comparative analysis of dendrogram of selected bacterial isolates based on (a) in vitro tested growth promoting ability and (b) 16S rRNA sequences. (Where, B.p. JRBHU1= Burkholderia paludism, B.p. JRBHU2= Burkholderia paludism, P.d. JRBHU3= Pantoea dispersa, P.l. JRBHU4= Pseudomonas lactis, P.a. JRBHU5= Pseudomonas azotoformans, B.s. JRBHU6= Burholderia seminalis, E.c. JRBHU7= Enterobacter cloacae, E.h. JRBHU8= Enterobacter hormaechei, E.h. JRBHU9= Enterobacter hormaechei, E.c. JRBHU10= Enterobacter cloacae, B.s. JRBHU11= Bacillus subtilis.)
While the 16S rRNA gene sequences formed 2 main clusters based on genetic similarity, the dendrogram based on PGP traits also formed 2 main groups. Strains of Pseudomonas JRBHU4 and JRBHU5 clustered together in both cases and showed high relatedness. Both strains also exhibited high mineral (P, K, and Zn) solubilization capacity and biocontrol activity by the production of siderophores, protease, cellulase, and chitinase. Similarly, all the Burkholderia strains JRBHU1, JRBHU2 and JRBHU6 clustered together based on their 16S rRNA gene sequence as well as PGP traits. The group represented by rhizobacteria (En. cloacae JRBHU7, En. hormaechei JRBHU8, En. cloacae JRBHU10), showed the highest extracellular enzyme production. The Enterobacter strains JRBHU7, JRBHU8, and JRBHU10 showed close similarity to Pantoea based on the phylogeny but based on their PGP traits it showed resemblance with Pseudomonas JRBHU4 and JRBHU5. A remarkably distinct clade formed by Bacillus in the phylogenetic tree was also found to be quite distinct in the dendrogram based on its PGP traits. The Pantoea strains JRBHU3 and JRBHU11 although belonging to the same genus were found to be distantly related in the dendrogram for the PGP traits.
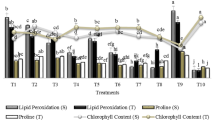
Effect of PGPR on plant growth parameters. Seed biopriming with bacterial strains possessing potential PGP traits could be a promising approach in integrated solutions to various environmental stresses because these strains not only help in enhancing nutrient availability and acquisition but also support plant’s growth and development under adverse conditions. Biopriming with selected PGPRs brought significant improvement in wheat growth parameters compared to the control plants. Percent seed germination after PGPR biopriming was 95% for strain Ps. azotoformans JRBHU5, 94% for Pa. dispersa JRBHU3 and Bu. seminalis JRBHU6 and 93% for En. hormaechei JRBHU9 and Ba. subtilis JRBHU11, while control showed 78% germination. Similarly, seedlings treated with Ps. azotoformans JRBHU5 showed the best increment of 71% in root length followed by Pa. dispersa JRBHU3 (67%), Bu. seminalis JRBHU6 (64%), En. hormaechei JRBHU8 (59%) and Ba. subtilis JRBHU11 (58%) with respect to control. It was also observed that Ps. azotoformans JRBHU5 showed the best increase in shoot length of 51.58%, followed by 47.22, 45.23, 43.05% increase observed in Bu. seminalis JRBHU6, En. hormaechei JRBHU9 and Ba. subtilis JRBHU11 inoculated plant with respect to control. Application of the other bacterial strains too showed moderate increase in root and shoot lengths. Significant differences in vigor index were also observed in plants as its maximum value was observed in Ps. azotoformans JRBHU5 (97.34%) followed by Bu. seminalis JRBHU6 (89.17%), Pa. dispersa JRBHU3 (84.34%), Ba. subtilis JRBHU11 (80.93%) and En. hormaechei JRBHU9 (78.30%), while Ps. lactis JRBHU4 (50.13%) treated plants had low vigor index compared to the control. Nevertheless, all plants treated with different isolates had appreciable vigor value above the control (Table 2). Further, the ratios of root/shoot lengths were also obtained and all the strains showed higher value as compared to control with En. cloacae JRBHU10 having the best value with respect to control. Seed biopriming with selected bacterial strains showed significant enhancement in fresh weight biomass of seedling with maximum increase observed in Ps. azotoformans JRBHU5 (165%) followed by Bu. seminalis JRBHU6 (154%), En. hormaechei JRBHU9 (149%), Pa. dispersa JRBHU3 (139%) and Ba. subtilis JRBHU11 (136%) when compared to uninoculated control. Almost a similar trend was observed in the seedling dry weight with maximum value observed in En. hormaechei JRBHU9 (144%) followed by Ps. azotoformans JRBHU5 (142%), Bu. seminalis JRBHU6 (139%) and Pa. dispersa JRBHU3 (134%) respectively as compared to uninoculated control.
When grown in pots under greenhouse conditions (Fig. 3) the PGPR treatments in wheat showed significant differences in the leaf area (LA), plant height (PH), days to heading (DH), days to maturity (DM), grains/spike (GPS), spike length (SL), thousand grains weight (TGW) but not for the number of tillers (NT). Seed biopriming with strain Ps. azotoformans JRBHU5 showed a significant and maximum increase in plant height (50%), spike length (24%) and number of grains/spike (63%) followed by Bu. seminalis JRBHU6, En. hormaechei JRBHU9, Ba. subtilis JRBHU11 and Pa. dispersa JRBHU3 as compared to the untreated control. Flag leaf area of wheat was significantly higher in treatments of Ps. azotoformans JRBHU5 (125%) followed by Pa. dispersa JRBHU3 (75.46%), Bu. seminalis JRBHU6 (72.35%) and En. hormaechei JRBHU9 (69.52%) as compared to untreated control plants. PGPR-treated plants had more days to heading and maturity when compared to control plants which showed early heading and seed setting as the vigor of these plants was low. Treatment with Ps. azotoformans JRBHU5 showed highest days to heading (79.33 days) and days to maturity (108.66 days) followed by Bu. seminalis JRBHU6, Pa. dispersa JRBHU3 and En. hormaechei JRBHU9 as compared to untreated control plants in which heading was observed after 69 days and grain maturity after 99 days. The 1000 grain weight represents the magnitude of seed development and reflects the crop yield. A longer duration in seed maturity offered ample time for grain development and storage as reflected in the higher yield. The 1000 grain weight was significantly higher in the plants treated with Ps. azotoformans JRBHU 5 (28.63%) followed by Bu. seminalis JRBHU6 (26.50%) and Pa. dispersa JRBHU3 (26.06%) as compared to untreated control (27.73 gm). All the selected PGPR’s in the present study showed significant role in plant growth by modifying the root system architecture through production of phytohormones, improving plant nutrition via nutrient mobilization and EPS secretion which influenced the physiology of the entire plant. However, the PGPR signals that could trigger the molecular pathways involved in the plant developmental responses remain to be studied. The EPS of PGPR’s having varied composition and functional groups influence a myriad of soil factors (biofilm, soil aggregation, water retention, nutrient locking) which in turn modulate PGPR effects on roots (Costa Oya et al., 2018). Interactions between the functional groups in the EPS such as uronic acids and acetyl groups and the protein adhesion enhance soil adhesion and biofilm formation thereby facilitating in chemical communication during the host plant interactions.
Pot trial of inoculated plants with selected bacterial isolates. (Where: B.p. JRBHU1 = Burkholderia paludism, B.p. JRBHU2 = Burkholderia paludism, P.d. JRBHU3 = Pantoea dispersa, P.l. JRBHU4 = Pseudomonas lactis, P.a. JRBHU5 = Pseudomonas azotoformans, B.s. JRBHU6 = Burholderia seminalis, E.c. JRBHU7 = Enterobacter cloacae, E.h. JRBHU8 = Enterobacter hormaechei, E.h. JRBHU9 = Enterobacter hormaechei, E.c. JRBHU10 = Enterobacter cloacae, B.s. JRBHU11 = Bacillus subtilis.)
An insight with the interrelationship between the plant growth-promoting traits and the external factors influencing the traits is of great value in crop improvement and therefore the correlation co-efficient which gives a measure of degree and the direction of association between the traits was studied in the present investigation correlating phenotypic wheat growth parameters with the bacterial growth promoting traits. The correlation matrix is presented in Fig. 4. Production of the microbial hormone IAA secreted exogenously was highly positively correlated to the plant growth parameters like root length (0.521), vigor index (0.505), thousand grains weight (0.561), and flag leaf area (0.404). It was however negatively correlated to the days to heading (−0.197), indicating that better vegetative growth of plants probably delayed the reproductive onset. The nutrient-solubilizing (P, K, Zn) abilities of the PGPR’s directly contributed to enhancing plant growth and vigor, as a highly significant and positive correlation was found with seedling fresh weight, root length, plant height and thousand grain weight. Among the nutrients, the phosphorus-solubilizing ability of the PGPR’s was more significantly correlated to such plant growth parameters as shoot length (0.743*), root length (0.781**), seedling fresh weight (0.786**), dry weight (0.661*), vigor index (0.753*) and plant height (0.669*) as compared to solubilization of the other nutrients, indicating the vital role of phosphorus in cell division, development of new tissue and its association with the complete energy requirements of the plants. The microbial extracellular polymeric substances contributing in the ecological functions and stress tolerance showed highly significant positive correlations with seedling root length (0.717*), seedling fresh weight (0.707*), vigor index (0.578), numbers of tillers per plant (0.575), and thousand grain weight (0.513). All the bacterial strains evaluated for growth promotion showed a highly significant positive correlation between the EPS and plant growth attributes. However, as the adhesiveness of exopolysaccharides is highly dependent upon the polymeric chain conformation, internal substitutes, and the internal/external interactions (Berne et al., 2015), therefore the extent to which each bacterial EPS has contributed to affecting plant growth attributes remains to be determined.
Correlations between various bacterial and plant growth parameters. (Where: IAA—IAA production, P—Phosphate solubilization, K—Potassium solubilization, Zn—Zinc solubilization, EPS—Exopolysaccharides production, PG—Percentage of germination, SL—Seedling shoot length, RL—Seedling root length, FW—Seedling fresh weight, DW—Seedling dry weight, VI—Vigor index, NT—Number of Tillers, FLA—Flag Leaf Area, PH—Plant height, DH—Days to 50% heading, DM—Days to 50% Maturity, GPS—Grains/Spike, SPL—Spike length, TGW—Thousand grains weight.)
These findings suggest that inoculating selected bacterial isolates in wheat brings remarkable improvement in plant growth through various mechanisms as also reported in similar earlier studies. Increase in the root length observed was up to 71% after inoculation of strain Ps. azotoformans JRBHU5 followed by Pa. dispersa JRBHU3 (67%), Bu. seminalis JRBHU6 (64%), En. hormaechei JRBHU8 (59%) and Ba. subtilis JRBHU11 (58%). Increased root length not only supports plant growth by exploring a greater volume of soil thereby increasing nutrient availability and absorption, but is also of great significance in plants exposed to drought stress as they increase water absorption in plants. It is worth noting that the increase in growth parameters observed on inoculation of PGPR strains usually has been found to increase the root length and root biomass (Khalid et al., 2004). Most of the plant growth parameters affecting crop yield were significantly affected by the PGPR treated. All the selected PGPR strains were able to significantly influence the numbers of tillers, flag leaf area, plant height, number of grains per spike and 1000 grain weight. Strain Ps. azotoformans JRBHU5 showed a more pronounced effect on growth (plant height and tillers/ plant) and yield contributing parameters (spike length, number of grains per spike, 1000 grain weight). Malik et al. (2012) also found enhanced germination counts, increases in numbers of tillers and flag leaf area, number of grains per spike and 1000-grains weight of wheat after inoculating Pseudomonas sp.
Thus, it may be concluded that PGPR strains showing multifaceted plant growth-promoting traits with excellent root colonizing ability and EPS production prove to be efficient in promoting the early vegetative and late reproductive growth parameters of wheat, and strongly inhibit the growth of phytopathogens. Although few PGPR’s capable of forming biofilm have been reported (e.g., Rhizobium leguminosarum, Agrobacterium sp., Azotobacter vinelandii, Enterobacter cloacae, Xanthomonas sp., Pseudomonas sp., Paenibacillus polymyxa, Bradyrhizobium sp., Bacillus subtilis and Bacillus drentensis) but not many are free living to be exploited on wider plant host (Gupta et al., 2017). Thus, the isolated strains Pseudomonas azotoformans JRBHU5 and Burkholderia seminalis JRBHU6 with good colonizing abilities are worthy bioinoculants for enhancing plant vigor and commercial production.
REFERENCES
Alexander, M., Introduction to soil microbiology, Soil Sci., 1978, vol. 125, p. 331.
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J., Gapped BLAST and PSI-BLAST: a new generation of protein database search Programs, Nucleic Acids Res., 1997, vol. 25, pp. 3389–3402.
Berne, C., Ducret, A., Hardy, G.G., and Brun, Y.V., Adhesins involved in attachment to abiotic surfaces by gram-negative bacteria, Microbial Biofilms, 2015, pp. 163–199.
Bramhachari, P.V. and Dubey, S.K., Isolation and characterization of exopolysaccharide produced by Vibrio harveyi strain VB23, Lett. Appl. Microbiol., 2006, vol. 43, no. 5, pp. 571–577.
Carson, K.C., Meyer, J.M., and Dilworth, M.J., Hydroxamate siderophores of root nodule bacteria, Soil Biol. Biochem., 2000, vol. 32, no. 1, pp. 11–21.
Cappuccino, J.G. and Sherman, N., Microbiology: A Laboratory Manual, San Francisco, CA, USA, 2008, 8th ed.
Coenye, T., Liu, L., Vandamme, P., and Li Puma, J.J., Identification of Pandoraea species by 16S ribosomal DNA-based PCR assays, J. Clin. Microbiol, 2001, vol. 39, no. 12, pp. 4452–4455.
Costa, O.Y.A., Raaijmakers, J.M., and Kuramae, E.E., Microbial extracellular polymeric substances: ecological function and impacts on soil aggregation, Front. Microbiol., 2018, vol. 9, p. 1636.
Dey, R. and Raghuwanshi, R., Comprehensive assessment of growth parameters for screening endophytic bacterial strains in Solanum lycopersicum (Tomato), Heliyon, 2020, vol. 6, no. 10, p. e05325.
Dubey, A., Saiyam, D., Kumar, A., Hashem, A., Abd_Allah, E.F., and Khan, M.L., Bacterial root endophytes: characterization of their competence and plant growth promotion in soybean (Glycine max (L.) Merr.) under drought stress, Int. J. Environ. Res. Publ. Health, 2021, vol. 18, no. 3, p. 931.
Elshafie, H.S., Camele, I., Racioppi, R., Scrano, L., Iacobellis, N.S. and Bufo, S.A., In vitro antifungal activity of Burkholderia gladioli pv. Agaricicola against some phytopathogenic fungi, Int. J. Mol. Sci., 2012, vol. 13, pp. 16291–16302.
Etesami, H., Emami, S., and Alikhani, H.A., Potassium solubilizing bacteria (KSB): mechanisms, promotion of plant growth, and future prospects: a review, J. Soil Sci. Plant Nutr., 2017, vol. 17, no. 4, pp. 897–911.
FAOSTAT statistical database, Food and Agriculture Organization of the United Nations, Rome, Italy, 2019.
Flaishman, M.A., Eyal, Z., Zilberstein, A., Voisard, C., and Haas, D., Suppression of Septoria tritici blotch and leaf rust of wheat by recombinant cyanide-producing strains of Pseudomonas putida, Mol. Plant-Microbe Interact., 1996, vol. 9, no. 7, pp. 642–645.
Flemming, H.C. and Wingender, J., The biofilm matrix, Nat. Rev. Microbiol., 2010, vol. 8, p. 623.
Gupta, S., Kaushal, R., Spehia, R.S., Pathania, S.S., and Sharma, V., Productivity of Capsicum influenced by conjoint application of isolated indigenous PGPR and chemical fertilizers, J. Plant Nutr., 2017, vol. 40, pp. 921–927.
ISTA, Proceeding of the international seed testing association, international rule for seed testing, Seed Sci. Technol., 1993, vol. 2, pp. 25–30.
Jochum, M.D., McWilliams, K.L., Borrego, E.J., Kolomiets, M.V., Niu, G., and Pierson, E.A., Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses, Front. Microbiol., 2019, vol. 10, p. 2106.
Khalid, A., Arshad, M., and Zahir, Z.A., Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat, J. Appl. Microbiol., 2004, vol. 96, pp. 473–480.
Kumar, A., Maurya, B.R., and Raghuwanshi, R., Isolation and characterization of PGPR and their effect on growth, yield and nutrient content in wheat (Triticum aestivum L.), Biocatal. Agric. Biotechnol., 2014, vol. 3, no. 4, pp. 121–128.
Malik, A.U., Malghani, A.L., and Hussain, F., Growth and yield response of wheat (Triticum aestivum L.) to phosphobacterial inoculation, Russ. Agricult. Sci., 2012, vol. 38, pp. 11–13.
Mosharaf, M.K., Tanvir, M.Z.H., Haque, M.M., Haque, M.A., Khan, M.A.A., and Molla, A.H., Metal-adapted bacteria isolated from wastewaters produce biofilms by expressing proteinaceous curli fimbriae and cellulose nanofibers, Front. Microbiol., 2018, vol. 9, p. 1334.
Naumann, D., FT-infrared and FT-Raman spectroscopy in biomedical research, Appl. Spectroscopy Rev., 2001, vol. 36, pp. 239–298.
Oleńska, E., Małek, W., Wójcik, M., Swiecicka, I., Thijs, S., and Vangronsveld, J., Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions, a methodical review, Sci. Total Environ., 2020, vol. 743, p. 140682.
Prasad, J.K., Gupta, S.K., and Raghuwanshi, R., Screening multifunctional plant growth promoting rhizobacteria strains for enhancing seed germination in wheat (Triticum aestivum L.), Int. J. Agric., 2017, vol. 12, no. 2, pp. 64–72.
Prasad, J.K., Pandey, P., Anand, R., and Raghuwanshi, R., Drought exposed Burkholderia seminalis JRBHU6 exhibits antimicrobial potential through pyrazine-1,4-dione derivatives targeting multiple bacterial and fungal proteins, Front. Microbiol., 2021, vol. 12, p. 513.
Premachandra, D., Hudek, L., Enez, A., Ballard, R., Barnett, S., Franco, C.M.M. and Brau, L., Assessment of the capacity of beneficial bacterial inoculants to enhance Canola (Brassica napus L.) growth under low water activity, Agronomy, 2020, vol. 10, no. 9, p. 1449.
Raghuwanshi, R. and Prasad, J.K., Perspectives of rhizobacteria with ACC Deaminase activity in plant growth under abiotic stress, in Root Biology. Soil Biology, Giri, B., Prasad, R., and Varma, A., Eds., Springer, 2018, vol. 52.
Sandhya, V.Z., Grover, M., Reddy, G., and Venkateswarlu, B., Alleviation of drought stress effects in sunflower seedling by the exopolysacchrides producing Pseudomonas putida strain GAP-P45, Biol. Fert. Soil., 2009, vol. 46, pp. 17–26.
Saravanan, V.S., Kumar, M.R., and Sa, T.M., Microbial zinc solubilization and their role on plants, in Bacteria in Agrobiology: Plant Nutrient Management, Berlin: Springer, 2011, pp. 47–63.
Sharma, M., Saleh, D., Charron, J.B., and Jabaji, S., A crosstalk between Brachypodium root exudates, organic acids, and Bacillus velezensis B26, a growth promoting bacterium, Front. Microbiol., 2020, vol. 11, p. 2432.
Sneath, P.H.A. and Sokal, R.R., Numerical Taxonomy. The Principles and Practice of Numerical Classification, San Francisco, Freeman, 1973.
Tamura, K., Stecher, G., and Kumar, S., MEGA 11: Molecular Evolutionary Genetics Analysis Version 11, Mol. Bi-ol. Evol., 2021.
Vardharajula, S., Exopolysaccharide production by drought tolerant Bacillus spp. and effect on soil aggregation under drought stress, J. Microbiol. Biotechnol. Food Sci., 2021, pp. 51–57.
Wakatsuki, T., Metal oxidoreduction by microbial cells, J. Ind. Microbiol., 1995, vol. 14, no. 2, pp. 169−177.
Wang, X., Sharp, C.E., Jones, G.M., Grasby, S.E., Brady, A.L., and Dunfield, P.F., Stable-isotope probing identifies uncultured Planctomycetes as primary degraders of a complex heteropolysaccharide in soil, Appl. Environ Microbiol., 2015, vol. 81, no. 14, pp. 4607–4615.
Yasmin, H., Naz, R., Nosheen, A., Hassan, M.N., Ilyas, N., Sajjad, M., Anjum, S., Gao, X., and Geng, Z., Identification of new biocontrol agent against charcoal rot disease caused by Macrophomina phaseolina in soybean (Glycine max L.), Sustainability, 2020a, vol. 12, no. 17, p. 6856.
Yasmin, H., Naeem, S., Bakhtawar, M., Jabeen, Z., Nosheen, A., and Naz, R., Halotolerant rhizobacteria Pseudomonas pseudoalcaligenes and Bacillus subtilis mediate systemic tolerance in hydroponically grown soybean (Glycine max L.) against salinity stress, PLoS One, 2020b, vol. 15, no. 4, p. e0231348.
ACKNOWLEDGMENTS
Jay Kishor Prasad would like to acknowledge the financial support as SRF (Grant no. 09/013(0810)-EMR-I), received from CSIR, Government of India New Delhi.
Author information
Authors and Affiliations
Contributions
Conceptualization: R. Raghuwanshi; J.K. Prasad, Methodology: J.K. Prasad; Formal analysis and investigation: J.K. Prasad; Writing: J.K. Prasad; R. Dey; Writing—review and editing: R. Raghuwanshi, R. Dey, J.K. Prasad, Funding acquisition: J.K. Prasad; Resources: R. Raghuwanshi; Supervision: R. Raghuwanshi.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.The authors declare that they have no conflicts of interest-.
DATA AVAILABILITY (DATA TRANSPARENCY):
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplementary Information
Rights and permissions
About this article
Cite this article
Prasad, J.K., Dey, R. & Raghuwanshi, R. Exopolysaccharide-Producing Rhizospheric Bacteria Enhance Yield via Promoting Wheat (Triticum aestivum L.) Growth at Early Stages. Microbiology 91, 757–769 (2022). https://doi.org/10.1134/S0026261721102622
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261721102622