Abstract
The aggregation behavior of new cationic hexadecyl surfactants with one or two alkylcarbamate fragments in the head group in water was studied using conductometry, spectrophotometry, fluorescence spectroscopy, and dynamic light scattering, and their catalytic action in hydrolytic processes was examined. Kinetic parameters of the alkaline hydrolysis of carboxylic acid esters (p-nitrophenyl acetate and p-nitrophenyl caprinate) were obtained upon the variation of the structures of surfactant head groups and the pH of solution. It was shown that the catalytic effect of micelle-forming surfactants with a single carbamate fragment is higher than that of the corresponding dicarbamate compounds, and it decreases with the alkyl chain length of substituents in head groups. It was found that carbamate surfactants capable of vesicle formation accelerate the hydrolysis of the test esters to a greater extent than their analogs forming micelles: the observed acceleration of the process can exceed two orders of magnitude in the case of a compound with the decyl substituent in a carbamate fragment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Compositions based on cationic surfactants are widely used in industry, agriculture, and medicine. They are successfully used as corrosion inhibitors, detergents, lubricants, supramolecular catalysts, antimicrobial and disinfecting agents, solubilizers, and drug carriers [1–5]. Their functional activity is based on the ability of surfactants to form nanosized aggregates in solutions, which effectively solubilize hydrophobic compounds and affect their reactivity, integrate into biomembranes, bind biologically active substances, and ensure their delivery to living organisms [6, 7]. The targeted design of amphiphilic molecules, the functionalization of their structure by introducing substituents that make it possible to involve various mechanisms of aggregation and binding of guest compounds, and the establishment of structure–property relationships are the ways that will help one to ensure a high application potential of surfactant-based systems and serve as the foundation for solving practically significant problems.
Solutions of cationic surfactants used as a reaction medium can strongly affect the properties of solubilized reagents, which make it possible to directionally control the rate and, in some cases, the mechanism of processes occurring in them [8–12]. The morphology of aggregates formed in surfactant solutions and the possibility of structural rearrangements induced by changes in concentration or external conditions have a significant effect on their functional properties [13, 14].
Cationic surfactants are traditionally used in micellar catalysis for the hydrolytic cleavage of ester bonds [15–18]. The catalytic effect is caused by the noncovalent binding of reactants with micelles mainly due to hydrophobic and electrostatic interactions, which lead to the concentration of reacting species in the dispersed phase and to a change in their microenvironment. The observed process acceleration strongly depends on the structure of the surfactant and on the presence of structural fragments that make it possible to use various mechanisms of reactant binding.
In this work, we continued our cycle of studies on cationic surfactants containing a carbamate fragment in the head group, which are capable of forming aggregates of various morphologies in a low concentration range and are distinguished by a high solubilization and membranotropic effect [19–22]. The possibility of using surfactant solutions of this class as a medium for hydrolytic processes is considered for the first time. In order to reveal the effect of the structure of hexadecyl mono- and dicarbamate surfactants on the ability to exert a catalytic effect, a comparative analysis of their aggregation behaviors in aqueous solutions and kinetic data characterizing the alkaline hydrolysis reactions of carboxylic acid esters in them was carried out.
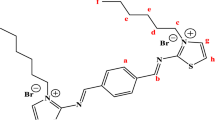
The range of test materials included some compounds obtained earlier along with new surfactants. The structural formulas of the test surfactants are presented below.

EXPERIMENTAL
The carbamate-containing compounds were synthesized by reactions of dimethyl(2-hydro-xyethyl)-hexadecylammonium bromide or methyl-di-(2‑hydroxyethyl)hexadecylammonium bromide with alkyl isocyanates using diazobicyclooctane (DABCO) as a catalyst according to a published procedure [19, 21]. The structure of the compounds was confirmed by IR and NMR spectroscopy, mass spectrometry, and elemental analysis data. Commercial samples of p-nitrophenol, p-nitrophenyl acetate, p-nitrophenyl caprinate, pyrene, and cetyltrimethylammonium bromide from Sigma-Aldrich were used in this study. Solutions were prepared using water purified on a Direct-Q 5 UV unit (Merck Millipore, France) (pH 6.8–7.0, χ = 2–3 μS/cm). Buffer solutions based on sodium tetraborate (pH 9.2) with the addition of sodium hydroxide (to pH 10.0) or hydrochloric acid (to pH 8.5) were used for kinetic experiments. The values of pH in the test solutions were monitored using a Hanna 211 laboratory pH meter (Hanna, Germany). The specific electrical conductivity of the samples was measured with an Inolab Cond 720 conductometer (WTW, Germany).
The sizes of obtained aggregates in solutions were determined on a Malvern ZetaSizer Nano photon correlation spectrometer for dynamic and electrophoretic light scattering (Malvern Instruments, the United Kingdom). The source of laser radiation was a He–Ne gas laser with a power of 10 mW and a wavelength of 633 nm. The light scattering angle was 173°. The pulse accumulation time was 5–8 min. Signals were analyzed using a single-board multichannel correlator connected to an IBM PC compatible computer equipped with a software package for estimating the effective hydrodynamic radius of dispersed particles.
The surface potential of the aggregates was estimated using a spectrophotometric method by studying changes in the acid–base properties of an indicator (p‑nitrophenol) depending on the surfactant concentration in accordance with published procedures [16, 23]. Spectral measurements were performed using a Specord 250 Plus spectrophotometer (Analytik Jena, Germany) with quartz cells with an optical path length of 1 cm at a temperature of 25°C.
The observed value of pKa,obs was calculated using the Henderson–Hasselbach equation
The value of the observed dissociation constant at Csurfactant → ∞ was taken as the dissociation constant of p-nitrophenol in the micellar phase (Ka,m).
The obtained values of pKa,m are related to the surface potential of a micelle by the equation
where pKa,0 is the nonelectrostatic component defined as pKa,obs of p-nitrophenol in micellar solutions based on nonionic surfactants (in Tween 80, probe pKa = 7.6); F = 96485 C mol–1 is the Faraday constant; R = 8.314 J K–1 mol–1 is the gas constant; and Ψ is the surface potential.
Fluorescence spectra of pyrene at a concentration of 1 × 10–6 mol/L in surfactant solutions were recorded using a Cary Eclipse spectrophotometer (Varian, the United States) at 25°С. Emission spectra were recorded in a range of 350–500 nm; the sample excitation occurred at a wavelength of 335 nm, the thickness of the absorbing layer was 1 cm, and the scanning rate was 120 nm/min.
The kinetics of alkaline hydrolysis of carboxylic acid esters was studied by spectrophotometry on a Specord-250 Plus instrument (Analytik Jena, Germany) using thermostatically controlled quartz cells. The course of the process was monitored by changes in the optical density of solutions at a wavelength of 400 nm, which corresponds to the absorption maximum of the p-nitrophenolate anion. The initial substrate concentration was (2–5) × 10–5 mol/L, and the degree of conversion was higher than 90%. The observed rate constants (kobs) were determined from the dependence ln(D∞ – D) = –kobst + const, where D and D∞ are the optical densities of the solution at the point in time t and at the end of the reaction, respectively. The values of kobs were calculated by the least squares method.
The error of all measurements did not exceed 4%.
RESULTS AND DISCUSSION
The first stage of studying the properties of carbamate surfactants traditionally included a study of their behavior in aqueous solutions and the establishment of concentration and temperature boundaries of aggregation, which predetermine the area of their functional activity. The values of critical aggregation concentration (CAC) and the Krafft temperature (TKr) were found by conductometry. As an example, Fig. 1 shows typical dependences that allow one to determine the CACs of the test compounds. Table 1 summarizes the results obtained for new surfactants in comparison with data for compounds synthesized earlier [15, 19]. In the case when micelles are formed in surfactant solutions, the term CAC is replaced by a more particular definition—the critical micelle concentration (CMC).
The CAC decreases with the length of an alkyl radical in the carbamate fragment and on going from mono- to dicarbamate derivatives. The values found for these compounds are significantly lower than the CMC of their trimethylammonium analogue, cetyltrimethylammonium bromide (CTAB). This is probably due to the ability of carbamate compounds to form hydrogen bonds [24], which facilitates the processes of their self-organization in aqueous solutions. The Krafft points of the carbamate surfactants CB-2-16, CB-4-16, and CB-2,2-16 are below 15°C (Table 1), which indicate the possibility of working with these compounds in a wide temperature range. The compounds CB-6-16 and CB-4,4-16 have a higher temperature threshold of micellization, but they are capable of aggregation at significantly lower concentrations than their analogs with a short alkyl substituent in the head group. The limited solubility of the compounds CB-8-16 and CB-10-16 in water did not allow us to reliably determine the values of TKr for them.
The degree of binding of counterions (β) by micelles was calculated from the concentration dependences of the electrical conductivity of carbamate surfactants (Fig. 1): β = 1 – α, where α is the degree of dissociation determined as a ratio between the slopes of linear sections before and after the point of change in the slope (Table 1). The values of β for the studied surfactants did not differ much from each other; however, they were significantly lower than that in the case of the reference compound CTAB. This suggests that, for carbamate surfactants, counterions compensate the charge of the head groups to a lesser extent, which should be reflected in lower aggregation numbers.
Using dynamic light scattering, we found that the monocarbamate surfactants CB-2-16, CB-4-16, and CB-6-16 in aqueous solutions above CACs form aggregates with a hydrodynamic diameter of 3–10 nm, which, as a rule, corresponds to the formation of spherical micelles (Fig. 2a). Similar results were obtained for the dicarbamate surfactants CB-2.2-16 and CB-4,4-16. In the case of CB-10-16, larger aggregates were detected in solutions. Their size remained almost unchanged in a wide range of concentrations and was 80–110 nm, while the polydispersity index varied in a range from 0.2 to 0.3 (Fig. 2b). The aggregate size of about 100 nm, which did not depend on surfactant concentration, is a characteristic sign of the appearance of vesicles in solutions. Previously, we also observed spontaneous formation of vesicles for other carbamate surfactants with a dodecyl radical in the head group and found that a change in the concentration of the compound CB-8-16 can initiate a transition from micelles to vesicles [21]. It can be expected that surfactants of the same family, which form aggregates of different morphology in solutions, will exhibit different catalytic effects when they are used as a reaction medium.
We tested the solutions of surfactants with a carbamate fragment in the processes of the alkaline hydrolysis of carboxylic acid esters. Kinetic experiments to study the hydrolysis of p-nitrophenyl acetate (PNPA) and p-nitrophenyl caprinate (PNPC) were carried out under conditions of changing the pH of the medium and the structure and concentration of surfactants. The reaction scheme of the process under study is presented below:

where R = CH3 (PNPA) or C9H19 (PNPC).
The main factors responsible for the catalytic action of surfactants in solutions and common to micelles and vesicles are the concentration of reagents in the reaction contact zone and the change in their microenvironment. In the processes of alkaline hydrolysis, the factor of concentration of hydroxide ions near the positively charged surface of an aggregate mainly depends on its surface potential, while the polarity of the microenvironment depends on the localization of a substrate and the hydrophobicity of an inner core. In micellar solutions, these two parameters are frequently determined by spectral probes. In the first case, p-nitrophenol is used, the pKa value of which changes significantly due to electrostatic interactions with a micelle and selective sorption of the anionic form of the probe near its surface [23]. In the second case, a hydrophobic fluorescent pyrene probe is used, which reacts finely to changes in the microenvironment in the course of its solubilization by micelles. The approaches used to characterize micellar systems were extended in this work to surfactant solutions, where the formation of vesicles is observed. By analogy with published data [23], we used the spectrophotometric method described in detail in the Experimental section to find the values of pKa,m of p-nitrophenol in the solutions of carbamate-containing test surfactants and Eq. (2) to determine the values of the surface potentials of aggregates (Table 2).
The values of Ψ found for carbamate surfactants were lower than those for the nonfunctionalized analog CTAB; in this case, an increase in the number of carbamate fragments in the head group of surfactants led to a greater decrease in the surface potential. This reduced the ability of carbamate surfactants to concentrate the hydroxide ion at the surface of aggregates and impaired their catalytic activity in alkaline hydrolysis processes compared to the micellar solutions of CTAB.
To characterize the micropolarity of the aggregates, we recorded the fluorescence spectra of pyrene in the solutions of carbamate-containing surfactants under conditions of varying their concentration. The intensity ratio between the first 373 nm (I1) and third 384 nm (I3) peaks of this compound [24–26], which is sensitive to polarity in the probe localization zone, was a parameter used to evaluate the effect of the medium. For pyrene dissolved in water, the ratio I1/I3 was 1.6. The ratio I1/I3 < 0.6 was noted when the probe was dissolved in nonpolar solvents. If pyrene was located in the surface layer of micelles, the ratio I1/I3 was in a range of 1.0–1.4 [26]. The course of dependence I1/I3 = f(Csurfactant) (Fig. 3) reflects the process of aggregate formation in solutions, and it is characterized by two segments with different slopes, the intersection point of which corresponds to the value of CAC (or CMC). The values obtained by the fluorescence method were in good agreement with the conductometric data (Table 1).
The gradual decrease in the ratio I1/I3 for monocarbamate surfactants with the length of an alkyl substituent in the head group and its decrease on going to dicarbamate derivatives reflect a decrease in the polarity of the microenvironment of pyrene solubilized by micelles (or vesicles). Similar changes in the polarity of aggregates can affect any other solubilized hydrophobic compound, in particular, carboxylic acid esters whose hydrolytic stability was supposed to be studied in this work. It can be expected that a change from the solutions of more hydrophilic surfactants to more hydrophobic ones will lead to a decrease in the polarity of the ester microenvironment, which will lead to a weakening of its reactivity in the processes of nucleophilic substitution.
Thus, the nature of changes in the surface potential and micropolarity of the aggregates allowed us to expect that surfactants with one carbamate fragment will be more effective catalysts of nucleophilic substitution processes than dicarbamate compounds, and their effect will be lower than that in the case of CTAB.
The results of the kinetic experiment were presented in the form of graphs reflecting changes in the observed rate constants (kobs) of the hydrolysis of carboxylic acid esters as functions of the concentrations of surfactants (Figs. 4 and 5). It is well known that, in aqueous solutions of surfactants used as a reaction medium, the reaction can simultaneously proceed both in the dispersed phase and in the dispersion medium. The contribution of each of the processes to the observed rate constant can change depending on the properties of the reactants. For PNPA, which is a more hydrophilic substrate, the rate of hydrolysis in an aqueous medium is high, and accelerations associated with the presence of surfactants are not so pronounced to allow one to draw conclusions on the influence of their structure on the reactivity of this ester. However, based on the experimental kinetic data (Fig. 4a), we can state that monocarbamate surfactants are more active than dicarbamate ones and CB-10-16 exhibits an early catalytic effect providing a maximum acceleration of the process at a concentration of 1 × 10–3 mol/L.
A different behavior was observed with PNPC (Fig. 4b), the hydrolytic stability of which depends to a much greater extent on the concentration and structure of the surfactant. The strong effect of surfactants on the properties of this micelle-forming substrate is explained by the formation of mixed aggregates in which PNPC exhibits an increased reactivity compared to individual solutions [27]. As expected, surfactants with one carbamate fragment had a greater catalytic effect than that of corresponding disubstituted analogs. For micelle-forming surfactants, the catalytic effect decreased with the length of an alkyl substituent in the carbamate fragment; however, surfactants capable of forming vesicles exhibited abnormally high catalytic activity. Thus, for example, at a surfactant concentration of 0.5 × 10–3 mol/L and pH 9.2, the value of kobs for CB-2-16 was 0.003 s–1, and it increased to 0.007 and 0.011 s–1, respectively, on going to CB-8-16 and CB-10-16 under the same conditions.
The dependences obtained for all studied surfactants have a form typical for micellar catalyzed processes—a rather sharp increase in the rate constant followed by a plateau (Figs. 4 and 5); this fact allowed us to apply the following equation of a pseudophase model of micellar catalysis to the analysis of kinetic data [28]:
where k0 and km (s–1) are the first-order rate constants in an aqueous medium and a micellar phase, respectively; KS (L/mol) is the substrate binding constant; and C is the total surfactant concentration minus the CMC. The ratio km/k0 is considered as a measure of the catalytic effect of the system.
Based on the experimental data, we can conclude that, although micelle-forming monocarbamate surfactants bind substrates almost as well as their dicarbamate analogs (compare the values of KS in Table 3), they are much more effective in alkaline hydrolysis of esters. This is probably due to their higher surface potential, which ensures the concentration of hydroxide ions in the reaction contact zone. In solutions where the formation of vesicles is possible (CB-8-16 and CB-10-16), in the case of PNPA, a stronger binding of the substrate was observed; it is most likely that this was a reason for the high catalytic effect. A pronounced substrate specificity of the process was traced: for PNPA, the acceleration in the presence of surfactants varied from a factor of 6 to 9, while it exceeded two orders of magnitude for PNPC.
It was somewhat unexpected that the catalytic effect of carbamate-containing surfactants was stronger than that of their nonfunctionalized analog CTAB, although the substrate binding constants for them are close and the surface potential is lower. It can be assumed that the presence of carbamate fragments in the test surfactants leads to the formation of hydrogen bonds between amphiphiles and a solubilized ester, which causes a redistribution of electron density in the substrate molecule, increases its reactivity, and thereby accelerates the process under study. Similar anomalous effects of functionalized surfactants on the reactivity of organic compounds were observed earlier [29].
The values of CMC found from the kinetic experiment (Table 3) are somewhat lower than those determined by the conductometric method. This was traced to a greater extent in the case of PNPC and can be associated with the possibility of the formation of mixed aggregates with the participation of surfactants and a micelle-forming substrate. Another possible reason for the lower values of CMC can be the presence of alkali or buffer salts, which can act as counterions and affect micelle formation processes, in the reaction solution.
The most important factor that makes it possible to influence the rate of reaction in surfactant solutions is a change in the pH of the medium. The response to a change in this parameter can depend on the morphology of aggregates in the solution. In this regard, we studied the kinetics of hydrolysis of PNPA in solutions of CB-2-16 and CB-8-16 with varying pH (Fig. 5, Table 3).
We found that the substrate binding constant in the case of both surfactants remained unchanged in the test range of pH (from 8.5 to 10.0), while the observed acceleration of the reaction, described by the ratio km/k0, depended on the morphology of aggregates formed in the solution. Thus, in the case of the micelle-forming surfactant CB-2-16, this characteristic remained almost unchanged at all pH values during the hydrolysis of PNPC, whereas it increased with the alkalinity of the medium in the solutions of CB-8-16, which is prone to vesicle formation. We did not go to the region of higher pH values because it is well known that compounds with a carbamate fragment can be hydrolytically cleaved to the corresponding carbamic acid and quaternary ammonium alcohols. This process proceeds rapidly under the action of enzymes [30]. Under laboratory conditions, the hydrolysis process in the absence of enzymes proceeds at a noticeable rate only in strongly alkaline media. Thus, it was found with the use of potentiometry, which detects the release of acid in the course of cleavage, and CB-4-16 as an example that it should be heated at 70°C in a 0.1 N solution of sodium hydroxide for more than 5 h in order to ensure a conversion of higher than 50%. Under milder conditions, the alkaline cleavage of the test surfactants could not be detected for a long time. In addition, we determined the rate constants of hydrolysis of PNPC at pH 9.2 in freshly prepared solutions of CB-4-16 and in the solutions stored for a day, a week, and a month. Differences in the observed rate constants of the process were insignificant; they were within the measurement error range and did not reflect any systematic trends associated with the instability of the surfactant.
CONCLUSIONS
Thus, as a result of this study, we expanded the scientific base that characterizes the properties of solutions of cationic carbamate surfactants. We found that the new studied hexadecyl derivatives of this class of surfactants have a low aggregation threshold, which decreases with the length of an alkyl radical in the carbamate fragment and on going from mono- to dicarbamate surfactants. We determined a significant catalytic activity of these compounds in the processes of ester hydrolysis, which can be regulated by varying the nature of the surfactant and the pH of the medium. We found that carbamate surfactants capable of forming vesicles accelerated the hydrolysis of the studied esters to a greater extent than their analogues that form micelles. The characteristics obtained can serve as the basis for the development of efficient nanoreactors that allow chemical processes to be carried out in aqueous solutions.
REFERENCES
Sanders, L., Cationic Surfactants: Properties, Uses and Toxicity, New York: Nova Science, 2016.
Devínsky, F., Pisárčik, M., snd Lukáč, M., Cationic Amphiphiles: Self-Assembling Systems for Biomedicine and Biopharmacy, New York: Nova Science, 2017.
Sar, P., Ghosh, A., Scarso, A., and Saha, B., Res. Chem. Intermed., 2019, vol. 45, p. 6021. https://doi.org/10.1007/s11164-019-04017-6
Morsy, S.M.I., Int. J. Curr. Microbiol. Appl. Sci., 2014, vol. 3, issue 5, p. 237.
Zhu, Y., Free, M.L., Woollam, R., and Durnie, W., Prog. Mater. Sci., 2017, vol. 90, p. 159. https://doi.org/10.1016/j.pmatsci.2017.07.006
Zakharova, L.Ya., Mirgorodskaya, A.B., Zhiltsova, E.P., Kudryavtseva, L.A., and Konovalov, A.I., Molecular Encapsulation: Organic Reactions in Constrained Systems, Brinker, U.H. and Mieusset, J.-L., Eds., Chichester: Wiley, 2010, p. 397.
Rangel-Yagui, C.O., Pessoa, A., Jr., and Tavares, L.C., J. Pharm. Pharm. Sci., 2005, vol. 8, p. 147.
Banerjee, M., Panjikar, P.C., Bhutia, Z.T., Bhosle, A.A., and Chatterjee, A., Tetrahedron, 2021, vol. 88, p. 132142. https://doi.org/10.1016/j.tet.2021.132142
Aboudiab, B., Tehrani-Bagha, A.R., and Patra, D., Colloids Surf. A, 2020, vol. 592, p. 124602.
Lorenzetto, A.T., Berton, G., Fabris, F., and Scarso, A., Catal. Sci. Technol., 2020, vol. 10, p. 4492. https://doi.org/10.1039/D0CY01062F
Shen, T., Zhou, Sh., Ruan, J., Chen, X., Liu, X., Ge, X., and Qian, Ch., Adv. Colloid Interface Sci., 2021, vol. 287, p. 102299. https://doi.org/10.1016/j.cis.2020.102299
Schmidt, F., Zehner, B., Korth, W., Jess, A., and Cokoja, M., Catal. Sci. Technol., 2020, vol. 10, p. 4448. https://doi.org/10.1039/D0CY00673D
Buurma, N.J., Curr. Opin. Colloid Interface Sci., 2017, vol. 32, p. 69. https://doi.org/10.1016/j.cocis.2017.10.005
Bélières, M., Chouini-Lalanne, N., and Déjugnat, C., RSC Adv., 2015, vol. 5, p. 35830. https://doi.org/10.1039/C5RA02853A
Al-Shamary, M.N., Al-Lohedan, H.A., Rafiquee, M.Z.A., El-Ablack, F., and Issa, Z.A., J. Saudi Chem. Soc., 2017, vol. 21, p. 193. https://doi.org/10.1016/j.jscs.2014.01.002
Mirgorodskaya, A.B., Valeeva, F.G., Kushnazarova, R.A. Lukashenko, S.S.,and Zakharova, L.Y., Kinet. Catal., 2021, vol. 62, p. 82. https://doi.org/10.1134/S0023158420060099
Muff, J., MacKinnon, L., Durant, N.D., Bennedsen, L.F., Rügge, K., Bondgaard, M., and Pennell, K.D., Environ. Sci. Pollut. Res., 2020, vol. 27, p. 3428. https://doi.org/10.1007/s11356-019-07152-0
Zhil’tsova, E.P., Ibatullina, M.R., Lukashenko, S.S., Kadirov, M.K., and Zakharova, L.Y., Kinet. Catal., 2020, vol. 61, p. 269. https://doi.org/10.1134/S0023158420010140
Mirgorodskaya, A.B., Kushnazarova, R.A., Lukashenko, S.S., Voloshina, A.D., Lenina, O.A, Zakharova, L.Ya., and Sinyashin, O.G., J. Mol. Liq., 2018, vol. 269, p. 203. https://doi.org/10.1016/j.molliq.2018.08.007
Mirgorodskaya, A.B., Kushnazarova, R.A., Lukashenko, S.S., and Zakharova, L.Ya., J. Mol. Liq., 2019, vol. 292, p. 111407. https://doi.org/10.1016/j.molliq.2019.111407
Kushnazarova, R.A., Mirgorodskaya, A.B., Lukashenko, S.S., Voloshina, A.D., Sapunova, A.S., Nizameev, I.R., Kadirov, M.K., and Zakharova, L.Ya., J. Mol. Liq., 2020, vol. 318, p. 113894. https://doi.org/10.1016/j.molliq.2020.113894
Kushnazarova, R.A., Mirgorodskaya, A.B., and Zakharova, L.Y., Russ. Chem. Bull., 2021, vol. 70, p. 585. https://doi.org/10.1007/s11172-021-3129-z
Mchedlov-Petrossyan, N.O., Pure Appl. Chem., 2008, vol. 80, p. 1459.
Bertrand, A., Lortie, F., and Bernard, J., Macromol. Rapid Commun., 2012, vol. 33, p. 2062.
Piñeiro, L., Novo, M., and Al-Soufi, W., Adv. Colloid Interface Sci., 2015, vol. 215, p. 1.
Lakowicz, J.R., Principles of Fluorescence Spectroscopy, New York: Springer, 2006.
Menger, F.M. and Portnoy, C.T., J. Am. Chem. Soc., 1968, vol. 90, p. 1875.
Berezin, I.V., Martinek, K., and Yatsimirskii, A.K., Usp. Khim., 1973, vol. 42, no. 10, p. 1729.
Kushnazarova, R.A., Mirgorodskaya, A.B., Kuznetsov, D.M., Tyryshkina, A.A., Voloshina, A.D., Gumerova, S.K., Lenina, O.A., Nikitin, E.N., and Zakharova, L.Ya., J. Mol. Liq., 2021, vol. 336, p. 116318. https://doi.org/10.1016/j.molliq.2021.116318
Mishra, S., Pang, Sh., Zhang, W., Lin, Z., Bhatt, P., and Chen, Sh., Chemosphere, 2021, vol. 279, p. 130500. https://doi.org/10.1016/j.chemosphere.2021.130500
Funding
This work was supported by the Russian Science Foundation (project no. 19-73-30012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Makhlyarchuk
Abbreviations and notation: DABCO, diazobicyclooctane; CAC, critical aggregation concentration; CMC, critical micelle concentration; CTAB, cetyltrimethylammonium bromide; PNPA, p-nitrophenyl acetate; and PNPC, p-nitrophenyl caprinate.
Rights and permissions
About this article
Cite this article
Mirgorodskaya, A.B., Kushnazarova, R.A., Kuznetsov, D.M. et al. Aggregation Behavior and Catalytic Action of Carbamate-Bearing Surfactants in Aqueous Solutions. Kinet Catal 63, 261–269 (2022). https://doi.org/10.1134/S0023158422030065
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422030065