Abstract
Flash photolysis of aromatic azides 4-R–C6H4N3 (where R = Ph, OPh, CH2Ph) in aerated acetonitrile solutions led to the intermediate formation of cis and trans isomers of the corresponding aryl nitroso oxides, which then presumably transformed via the aromatic ring opening into R-substituted unsaturated nitrile oxides. The electronic spectra of the cis and trans isomers of 4-R–C6H4NOO were recorded; the effective rate constants of the monomolecular decay of the isomers were determined, and the activation parameters of the rate constants were found. The rate constants ktrans are significantly lower than kcis, slightly varying in the series of nitroso oxides in the range 0.15–0.22 s–1 (295 K). The rate constants kcis for phenyl- (2.5 s–1) and benzyl- (2.8 s–1) substituted aryl nitroso oxides coincide within the experimental error, whereas the phenoxy substituent leads to an increase in kcis by a factor of ~1.5 (4.3 s–1). The decay of 4-R–C6H4NOO is represented by a system of three elementary reactions, including reversible isomerization of the cis and trans isomers and ortho-cyclization of cis-nitroso oxides into the corresponding nitrile oxide. Solving the system of differential equations describing the decay kinetics of 4-R–C6H4NOO led to an analytical time dependence of optical density of the reaction mixture, which coincides with the empirical dependence found earlier. This made it possible to reveal the physical meaning of the effective rate constants and calculate the elementary rate constants of the decay of aromatic nitroso oxides. The DFT calculations of activation barriers for cis-trans isomerization and irreversible ortho-cyclization of cis-4-R–C6H4NOO are in good agreement with experiment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Nitroso oxides, labile species of the general formula RNOO from the class of 1,3-dipolar peroxide species X–O–O, are intermediates in the thermal and photochemical reactions involving triplet nitrenes and occurring in the presence of molecular oxygen. The unique properties of nitroso oxides have been studied in recent years and were considered in reviews devoted to organic azides and nitrogen- and oxygen-containing heterocyclic compounds [1–5]. The structure, spectral properties, generation methods, reactivity, and mechanisms of various transformations of aromatic nitroso oxides ArNOO were considered in detail in [6–13].
It was found that simple nitroso oxide HNOO has a planar structure and exists in the form of two stable stereoisomers, trans and cis [14]:

The electronic structure of HNOO in the ground state is characterized by a three-center 4π electron system. Additional π bonding leads to an increase in the N–O and O–O bond order and a decrease in the interatomic distances by ~0.15 Å compared to those of the single bonds [15]. In the isomers of HNOO, the ratio of the bond lengths of the nitroso oxide fragment is inverted: in the trans isomer, r(NO) > r(OO), while for the cis form, the ratio is inverted because of the anomeric stabilizing interaction nN → \(\sigma _{{{\text{OO}}}}^{*},\) which is possible only in the cis isomer. For the same reason, the cis form of HNOO is thermodynamically more stable than the trans form by 8.1–10.9 kJ/mol [16].
The presence of an aromatic substituent in ArNOO conjugated with the 4π electron nitroso oxide group significantly stabilizes the latter. Like the simplest nitroso oxide, aryl nitroso oxides also exist in two planar (cis and trans) conformations, which are close in energy. The cis and trans states of ArNOO are separated by a significant conformation barrier [17] due to the partial π bonding between the N and O atoms. The relatively low rate of the conformational cis–trans transition explains the experimental fact, according to which cis- and trans-ArNOO behave as kinetically independent species that differ in the chemical nature and reactivity. According to theoretical estimates [18], the optical absorption spectrum of HNOO is characterized by a maximum at a wavelength of ~260 nm. The formation of a common π electron system markedly shifts the absorption band in the electronic spectra of aromatic nitroso oxides to the long-wave region. The high polarity and dynamicity of the π electron system of aromatic nitroso oxides explains the solvent effect on the distribution of electron density: it was shown [19] that the density increases on the nitroso oxide fragment and the dipole moment of the cis–trans isomers of the 4-R–C6H4NOO series increases with the dielectric permittivity in the series gas phase–n-heptane–benzene–acetonitrile.
As nitroso oxides are short-lived intermediates, they were detected for the first time only in 1971 using matrix isolation combined with spectral methods [20]. Today, another method is successfully used at our laboratory to study the isomeric forms of these labile species, namely, flash photolysis with time-resolved spectrophotometry. The use of this method enabled us to identify isomers for a number of aryl nitroso oxides in media of different polarities at room temperature and higher temperatures. The decay of ArNOO, in contrast to that of related aryl carbonyl oxides Ar2COO, occurs according to the first-order kinetic law [8] even when there is no substrate of oxidation. The observed kinetic law of the decay of ArNOO was explained using photo-oxidation of 4-methoxyphenyl azide 1 in an acetonitrile solution as an example [9]. It was proved that a nontrivial intramolecular reaction occurs [21], in which the terminal oxygen atom of the cis form of nitroso oxide 2a is coordinated at the ortho-carbon atom of the aromatic ring (Scheme 1), forming bicyclic dioxazole. Whether this compound is a stable structure or a transition state is currently unknown. There is no information on the spectral identification of dioxazole, and theoretical estimates give ambiguous results: calculations by the G2MP2B3 method indicate the presence of an activation barrier with a height of 15 kJ/mol for dioxazole decomposition [22], while no minimum corresponding to dioxazole was found on the potential energy surface (PES) of the reaction system when modeling in the M06L/6-311 + G(d,p) approximation [23]. In any case, the opening of the metastable dioxazole ring at the C–C and O–O bonds leads to nitrile oxide 3. Obviously, only the cis isomer of nitroso oxide can undergo ortho-cyclization. Nevertheless, the authors of [10] showed that both isomers of nitroso oxide 2 are converted into product 3; i.e., the main channel of the decay of 2b is its trans–cis isomerization to 2a:

Scheme 1 . Mechanism of photo-oxidation of aromatic azides.
The stability of the resulting nitrile oxide 3 changes depending on the electronic properties of the R substituent in the aromatic ring of the starting nitroso oxide. The relatively stable nitrile oxide (R = OMe) was identified directly by spectrometry [10]. In other cases, the intermediate formation of 3 was recorded according to the stable products of its subsequent intramolecular transformations [12, 23] and (3 + 2) cycloaddition to the acetonitrile solvent [13]. The driving force of these transformations is the presence of a reaction site—a multiple bond or a lone electron pair at which the electrophilic attack of nitrile oxide occurs with the formation of heterocyclic oximes [23], benzisoxazoles [12], and oxadiazoles [13]. The aromatic substituent in nitroso oxide also represents a potential reaction site for an attack by nitrile oxide, but this possibility has not yet been studied. For this reason, the objects of study here were azides Ia–Ic as their photo-oxidation should lead to nitroso oxides IIa–IIc, whose structure is of interest to us. However, the reactivity of IIa–IIc in ortho-cyclization has not been studied. The effect of the properties and position of the R substituent in the benzene ring on the activation barrier ΔH≠ of the intramolecular ortho-cyclization of aromatic nitroso oxides was systematically studied theoretically in [21, 24]. As is known, the presence of a substituent in the ortho position significantly affects the activation enthalpy of ortho-cyclization [24], whereas the role of the para substituent is not so obvious [21]. Therefore, here we studied the effect of the substituent in the para position on the spectral characteristics of the isomeric forms of aromatic nitroso oxides 4-R–C6H4NOO (IIa–IIc) and on their kinetic behavior and thermodynamic parameters using flash photolysis of aryl azides 4-R–C6H4N3 (where R = –Ph, –ОPh, –CH2Ph) (Ia–IIc) in acetonitrile solutions. The results were compared with the corresponding parameters for other previously studied aromatic nitroso oxides.

EXPERIMENTAL
Acetonitrile (Kriokhrom) for high-performance liquid chromatography (HPLC) was used without preliminary purification. Aryl azides were synthesized according to [25].
The kinetic studies were performed on a flash photolysis unit of known design ENREF-7 [26]. The photolytic source was an IFP 5000-2 lamp (RadioTekhIndustriya, Russia); the maximum pulse energy was 400 J at U = 5 kV and C = 32 μF; ~90% of light energy is emitted in 50 μs. The spectrophotometric part of the unit consisted of a probing source of continuous radiation—a DKSSh-150 xenon lamp (Zapadpribor, Russia) with a system of quartz lenses and diaphragms for forming a probing beam; an MDR-4 monochromator (TD Labor, Russia); an FEU-97 photomultiplier (Zapadpribor, Russia); and an S9-8 storage oscilloscope (Kalibr plant, Minsk, Belarus). This unit also had a device for computer processing of the pulse signal. The signal from the photomultiplier is amplified after preliminary compensation of the DC component and fed to the input of the digital oscilloscope operating in a standby mode. The oscilloscope allows storage of a signal (2048 points) with a minimum time resolution of 50 ns and voltage resolution of 256 levels. The signal was digitized via the GPIB interface (IEEE-488, GPIB). The kinetic curves were processed by nonlinear regression analysis. The error in determining the rate constants was no more than 10%. The reactor was a thermostatted quartz cell with an optical length l = 10 cm and inner diameter of ~1 cm. The optical spectra of the para-substituted phenyl azides Ia–Ic studied by us have some features that are similar to those of the spectra of other phenyl azides [27]: the main maximum at 250–260 nm (log ε ≥ 4) and a wide shoulder at 280–300 nm (log ε ≥ 3), which attenuates near ~360–380 nm. Flash photolysis of aryl azide solutions in acetonitrile was performed with filtered light (UFS-2 light filter, transmission range λ = 270–380 nm). The initial concentration of azides was (1–2) × 10–4 M. To reduce the rate of decomposition of azides under the action of the probing beam, the region of their absorption was cut out with a CC-15 light filter, transmitting light in the wavelength range 300–520 nm, and the probing lamp was covered with a special shutter when preparing the measurement. It was shown by idle experiments that at the indicated concentrations of ArN3, the decomposition of azide is insignificant within the time of experiment (a few seconds) and does not introduce errors in the results. The activation parameters of the reaction were determined in the temperature range 277–333 K.
All DFT calculations were performed using the M06L density functional [28] and the 6-311 + G(d,p) split-valence triple zeta basis set [29, 30]. It was shown [17] that this level of theory adequately reproduces the geometrical structure of aromatic nitroso oxides, their equilibrium and nonequilibrium energy parameters, and the vibrational spectra of ArNOO. The quantum chemical calculations were performed on the cluster supercomputer of the Ufa Institute of Chemistry, Ural Federal Research Center, Russian Academy of Sciences, using the Gaussian 09, Revision C.1 software package [31].
RESULTS AND DISCUSSION
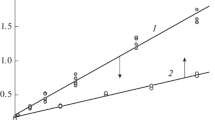
Flash photolysis of the acetonitrile solutions of azides Ia–Ic in the presence of atmospheric oxygen produces the cis and trans isomers of the corresponding nitroso oxides IIa–IIc, whose optical absorption spectra are recorded in the wavelength range 380–500 nm. The observed signal decreases to some constant value within a few seconds (Fig. 1). In the range of spectral wavelengths in which light is absorbed by both isomers, the kinetic curves of the decrease in optical density include the fast and slow sections (curve 1, Fig. 1): they correspond to the decay of both forms of ArNOO, which differ in reactivity, and are well described by the five-parameter biexponential equation (1):
where kcis and ktrans are the effective rate constants of the monomolecular decay of cis- and trans-nitroso oxide, respectively; A∞ is the optical density at the end of the reaction including the formation of reaction products. In the long-wave region of the given spectral range, the kinetic curves consist only of the slow component of Eq. (1) (curve 2, Fig. 1). In this region, the optical absorption of the cis isomer is negligible. Thus, the kinetic curves correspond to the decay of only the trans isomer and are well described by the first-order kinetic equation:
Typical kinetic curves of the decrease in the differential optical density of the solution of Ic ([Ic]0 = 2 × 10–4 M) after irradiation with light from a flash lamp, recorded at wavelengths of (1) 380 and (2) 460 nm and their approximation (solid lines) by Eqs. (1) and (2), respectively; T = 295 K, acetonitrile. Below are the deviations of the approximating function from the experimental values of the optical density R for both kinetic curves.
The electronic absorption spectra of isomers IIa–IIc (Fig. 2) were determined from the calculated A0(λ) values provided that the generation of ArNOO and tracing the optical density at all wavelengths are performed under identical conditions (azide concentration, light pulse energy). The shape of the optical spectra of IIa–IIc and the position of the absorption maxima correspond to the previously established tendencies for other aryl nitroso oxides [8] and to the theoretical calculations [12], according to which the absorption spectra of the trans isomer of nitroso oxides are shifted to the long-wave region compared to the spectra of the cis isomer. The λmax values for IIa–IIc are presented in Table 1, which also contains the data of [8] on the absorption maxima of the isomer forms of phenyl nitroso oxide (IId), 4-methyl- (IIe), and 4‑methoxyphenyl nitroso oxide (IIf) for comparison. In the series –H < –Me < –Ph, a bathochromic shift of the absorption maxima of the cis isomers of ArNOO on passing from unsubstituted phenyl nitroso oxide IId to IIa is observed. For the trans isomers, the bathochromic shift of λmax is even more pronounced. The observed order is appropriate within the framework of color theory: the energy of the electronic π → π* transition responsible for the absorption band of arylnitroso oxides in the visible region of the spectrum decreases when the conjugation region in IIa increases. The violation of conjugation due to the bridging methylene group –CH2–Ph in nitroso oxide IIc leads to a reverse shift of the absorption maximum to a value close to λmax for methyl-substituted ArNOO IIe. The oxygen bridge (–O–Ph) in IIb also breaks the conjugation between the two benzene rings located in the perpendicular planes (M06L calculation). However, due to the lone electron pairs, the oxygen atom donates electron density to the π system of aromatic nitroso oxide. The strong donor effect of the –OPh and –OMe substituents causes a significant bathochromic shift by 40–50 nm of the absorption maxima of cis isomers IIb and IIf and by 50–55 nm for the trans isomers compared to simple aryl nitroso oxide IId.
It is noteworthy that the ratio \({{A_{0}^{{{\text{trans}}}}} \mathord{\left/ {\vphantom {{A_{0}^{{{\text{trans}}}}} {A_{0}^{{{\text{cis}}}}}}} \right. \kern-0em} {A_{0}^{{{\text{cis}}}}}}\) in the absorption maxima of IIa–IIc markedly changes depending on the nature of the para-substituent (Fig. 2). A similar tendency was observed for nitroso oxides IId–IIf in [8]. For unsubstituted nitroso oxide and ArNOO with C-centered substituents, this ratio is large (~3–5). In the optical spectra of IIb and IIf, \(A_{0}^{{{\text{cis}}}}\) is ~80% of \(A_{0}^{{{\text{trans}}}}.\) The \({{A_{0}^{{{\text{trans}}}}} \mathord{\left/ {\vphantom {{A_{0}^{{{\text{trans}}}}} {A_{0}^{{{\text{cis}}}}}}} \right. \kern-0em} {A_{0}^{{{\text{cis}}}}}}\) ratio is evidently affected by the initial concentrations of the cis- and trans- isomers of ArNOO generated during the photolysis of azides and by the extinction coefficients of the isomers. It is also necessary to take into account the complex kinetic scheme of the decay of nitroso oxides (Scheme 1), according to which the simultaneous occurrence of reversible cis–trans isomerization and irreversible ortho-cyclization can have a significant effect on the calculated values of the pre-exponential terms in Eq. (1). The nature of the observed effect is currently studied in our laboratory.
The effective rate constants for the decay of nitroso oxide isomers IIa–IIc, determined using Eqs. (1) and (2) and presented in Table 1, demonstrate tendencies similar to those previously found for IId–IIf and some other aryl nitroso oxides. The rate constants ktrans are approximately an order of magnitude lower than kcis, change slightly in the series of nitroso oxides under study, and lie in the range 0.15–0.22 s–1 (295 K). The rate constants kcis for IIa and IIc coincide within the experimental error, and the phenoxy substituent leads to an increase in kcis by a factor of ~1.5–2. Table 1 also gives the results for the temperature dependences of the rate constants of the decay of cis and trans conformers IIa–IIc and the literature data for IId and IIf. It can be seen that the activation energies of the decay of both cis and trans isomers change in the same range of values, 60–70 kJ/mol, and the above-mentioned difference in the reactivity of the isomers is determined primarily by the value of the pre-exponential factor in the Arrhenius equation. It is interesting to note that for both isomers of aryl nitroso oxides, the dependence between logA and the activation energies is close to linear (the correlation coefficient is 0.98 in both cases) (Fig. 3). The compensation effect is associated with the fact that the trans isomer decays in the same way for all nitroso oxides under study; i.e., it turns into the cis isomer as a result of the conformational rotation at the N–O bond. Similarly, all cis isomers IIa–IIf undergo irreversible transformation into the corresponding nitrile oxide according to Scheme 1.
Indeed, recently [32], the mechanism of monomolecular decay of para-substituted aryl nitroso oxides was shown to be general using photo-oxidation of unsubstituted phenyl azide and its para-substituted analogs with electron-donating (MeO) and electron-accepting (Cl, Br) substituents as an example. Along with the main products of conversion of ArNOO—nitrile oxides or the products of their further transformation, relatively small amounts of nitro (ArNO2) and nitroso compounds (ArNO) are usually recorded in the reaction mixture [12, 13, 23, 32]. We attribute their formation to the photo-induced side reactions of aryl nitroso oxides. Kinetically these reactions can manifest themselves in slightly increased observed rate constants kcis and ktrans, and can be neglected within the experimental error. Thus, the formal kinetic scheme of the decay of aromatic nitroso oxides IIa–IIf is reduced to three stages with elementary rate constants kt, k–t, and kc (Scheme 2):

Scheme 2 . Kinetic scheme of the decay of aromatic nitroso oxides.
As all the reactions are monomolecular transformations, for the system of differential equations corresponding to Scheme 2, there is an analytical solution that has the form of a weighted sum of exponentials [17]. Passing from concentrations to optical densities, it is easy to obtain an analytical five-parameter dependence A(t), which coincides in form with the empirically selected equation (1). The analytical solution allows us to give strict physicochemical interpretation of the observed rate constants kcis and ktrans (Eqs. (3)–(6)):
Using Eqs. (3)–(6) it is possible to calculate the elementary rate constants from the experimentally determined kcis and ktrans. An unambiguous solution, however, requires an additional condition that correlates the elementary rate constants with the functional dependence. This condition is an expression for the equilibrium constant of cis–trans isomerization. In accordance with the Van’t Hoff isotherm equation
ΔG° equals the difference between the standard Gibbs energies of the cis and trans isomers of ArNOO, \(G_{{\text{c}}}^{^\circ }\) – \(G_{{\text{t}}}^{^\circ }.\) These values were calculated quantum-chemically in the M06L/6-311 + G(d,p) approximation, which ensures reliable calculation of the energy characteristics of aromatic nitroso oxides with an accuracy of high-level CCSD(T) calculations [17].
To find the elementary rate constants of stages from the effective constants, Eqs. (3)–(7) were transformed into the system of equations (8):
Of the three algebraic equations with three unknowns, it is easy to obtain a quadratic equation with respect to one of the elementary rate constants. The positive root of the quadratic equation is the desired rate constant, substituting which into the equations of system (8), we found the remaining constants. The elementary rate constants kt, k–t, and kc were calculated for all nitroso oxides over the entire temperature range of our experiments. The temperature dependence of the equilibrium constant ΔG° = ΔH° – TΔS° was included in calculation in the approximation of constant enthalpy and entropy of cis-trans isomerization. The calculated constants of the elementary stages are presented in Table 2.
The calculated elementary rate constants describing the decay of aromatic nitroso oxide isomers allow us to draw some conclusions. First, ortho-cyclization wins the competition and prevails over the isomerization of the cis isomer to the trans form, kc \( \gg \) k–t. As kc \( \gg \) kt, the expressions for the effective rate constants are significantly simplified: ktrans ≈ kt, kcis ≈ kc + k–t ≈ kc. The contribution of the isomerization of the cis isomer to the total rate of its decay is small in nitroso oxides IIb, IIc, and IIf; in IIa and IIe, it is ~10%; and only in IId it reaches ~20%. For this reason, the observed activation energies Ecis and Etrans are close to the activation energies of the corresponding elementary stages. Secondly, the theoretical estimates of the activation barriers of the stages of Scheme 2 indicate that ΔH≠ of cis–trans isomerization are determined quite reliably: the observed deviation from the experimental data is random, and the average absolute deviation is 4.6 kJ/mol. The energy barrier of the ortho-cyclization of IIa–IIf is systematically overestimated by an average of 8.7 kJ/mol in calculations in the M06L/6-311 + G(d,p) approximation. Although the absolute value of the error is small, the theory predicts that the ratio of rate constants (kt, k–t > kc) will be different from the experimentally observed ratio (kt, k–t < kc). This circumstance should be taken into account further in the theoretical analysis of the reactivity of aromatic nitroso oxides.
CONCLUSIONS
The cascade transformations occurring during photo-oxidation of aromatic azides and including a chain of highly active intermediates: nitrene–nitroso oxide–nitrile oxide lead to the formation of various heterocyclic structures [12, 13, 23, 32] depending on the structure of the starting aryl azide, in particular, on the nature of substituent in the aromatic ring. The aromatic system can be a potential trap for the nitrile oxide function; therefore, here we studied the kinetics of the decay of nitrile oxide precursors—cis and trans isomers of nitroso oxides IIa–IIc containing an aromatic substituent in the para position. The rate constants of the reversible isomerization of the trans conformers to the corresponding cis form and of ortho-cyclization of the cis isomers, forming nitrile oxides, were determined. The presence of a phenyl fragment in the substituent affects the reactivity of the isomers of ArNOO, as indicated by a comparison of the effective rate constants for the compounds and nitroso oxides that are structurally similar but contain aliphatic substituents (Table 1). However, the observed effect is insignificant, which generally agrees with the conclusions of [24] made on the basis of DFT modeling of ortho-cyclization of some substituted aryl nitroso oxides. The three-stage mechanism of the decay of ArNOO makes it possible to obtain an analytical solution to the system of differential equations describing the joint decay of the cis and trans isomers. This circumstance significantly expands prospects for kinetic analysis of the reactivity of ArNOO, in particular, for calculation of elementary rate constants for all stages of the mechanism of nitroso oxide decay using experimental rate constants (Table 2). Another possibility is currently being examined in our laboratory, namely, the use of spectral and kinetic data of flash photolysis measurements for separate determination of the extinction coefficients of ArNOO isomers.
REFERENCES
Gritsan, N.P. and Pritchina, E.A., Russ. Chem. Rev., 1992, vol. 61, no. 5, p. 500.
Gritsan, N.P., Russ. Chem. Rev., 2007, vol. 76, no. 12, p. 1139.
Ishiguro, K. and Sawaki, Y., Bull. Chem. Soc. Jpn., 2000, vol. 73, p. 535.
Sawwan, N. and Greer, A., Chem. Rev., 2007, vol. 107, no. 7, p. 3247.
Slayden, S.W., Greer, A., and Liebman, J.F., In The Chemistry of Hydrxylamines, Oximes and Hydroxamic Acids, Rappoport, Z. and Liebman, J.F., Eds., New York: Wiley, 2011, vol. 2.
Chainikova, E.M., Khursan, S.L., and Safiullin, R.L., Dokl. Phys. Chem., 2003, vol. 390, p. 163.
Chainikova, E.M., Khursan, S.L., and Safiullin, R.L., Dokl. Phys. Chem., 2005. 403, p. 133.
Chainikova, E.M., Safiullin, R.L., and Khursan, S.L., Kinet. Catal., 2006, vol. 47, no. 4, p. 549.
Chainikova, E.M., Safiullin, R.L., Teregulova, A.N., Spirikhin, L.V., and Galkin, E.G., Dokl. Chem., 2012, vol. 442, p. 30.
Chainikova, E.M., Safiullin, R.L., Spirikhin, L.V., and Abdullin, M.F., J. Phys. Chem. A, 2012, vol. 116, no. 31, p. 8142.
Chainikova, E.M., Pankratyev, E.Y., Teregulova, A.N., Gataullin, R.R., and Safiullin, R.L., J. Phys. Chem. A, 2013, vol. 117, no. 13, p. 2728.
Chainikova, E.M., Yusupova, A.R., Khursan, S.L., Teregulova, A.N., Lobov, A.N., Abdullin, M.F., Enikeeva, L.V., Gubaydullin, I.M., and Safiullin, R.L., J. Org. Chem., 2017, vol. 82, no. 15, p. 7750.
Chainikova, E.M., Khursan, S.L., Yusupova, A.R., Teregulova, A.N., Abdullin, M.F., Lobov, A.N., and Safiullin, R.L., J. Org. Chem., 2020, vol. 85, no. 16, p. 10813.
Nakamura, S., Takahashi, M., Okazaki, R., and Morokuma, K., J. Am. Chem. Soc., 1987, vol. 109, no. 14, p. 4142.
Talipov, M.R., Khursan, S.L., and Safiullin, R.L., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 3, p. 364.
Talipov, M.R., Khursan, S.L., and Safiullin, R.L., J. Phys. Chem. A, 2009, vol. 113, no. 23, p. 6468.
Yusupova, A.R., Safiullin, R.L., and Khursan, S.L., J. Phys. Chem. A, 2016, vol. 120, no. 28, p. 5693.
Talipov, M.R., Safiullin, R.L., Ryzhkov, A.B., and Khursan, S.L., J. Struct. Chem., 2006, vol. 47, no. 6, p. 1051.
Talipov, M.R. and Khursan, S.L., Vestn. Bashkir. Univ., 2005, vol. 10, no. 2, p. 42.
Brinen, J.S. and Singh, B., J. Am. Chem. Soc., 1971, vol. 93, no. 24, p. 6623.
Talipov, M.R., Khursan, S.L., and Safiullin, R.L., Russ. J. Phys. Chem. A, 2012, vol. 86, no. 2, p. 235.
Chainikova, E., Khursan, S., Lobov, A., Erastov, A., Khalilov, L., Mescheryakova, E., and Safiullin, R., Tetrahedron Lett., 2015, vol. 56, no. 32, p. 4661.
Chainikova, E., Khursan, S., Yusupova, A., Lobov, A., Abdullin, M., and Safiullin, R., Tetrahedron Lett., 2018, vol. 59, no. 34, p. 3267.
Yusupova, A.R., Chainikova, E.M., Safiullin, R.L., and Khursan, S.L., Int. J. Quantum Chem., 2020, vol. 120, no. 4, p. e26094.
Hu, M., Li, J., and Yao, S.Q., Org. Lett., 2008, vol. 10, no. 24, p. 5529.
Maslennikov, S.I., Nikolaev, A.I., and Komissarov, V.D., Kinet. Katal., 1979, vol. 20, p. 326.
UV/Visible Spectra by Talrose, V., Yermakov, A.N., Usov, A.A., Goncharova, A.A., Leskin, A.N., Messineva, N.A., Trusova, N.V., Efimkina, M.V., Eds., NIST Chemistry WebBook, NIST Standard Reference Database Number 69, Gaithersburg: National Institute of Standards and Technology, 2021). https://doi.org/10.18434/T4D303
Zhao, Y. and Truhlar, D.G., J. Chem. Phys., 2006, vol. 125, no. 19. 194101.
Wachters, A.J.H., J. Chem. Phys., 1970, vol. 52, no. 3, p. 1033.
McLean, A.D. and Chandler, G.S., J. Chem. Phys., 1980, vol. 72, no. 10, p. 5639.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B.G., et al., Gaussian 09, Wallingford, 2009.
Chainikova, E.M., Abdullin, M.F., Lobov, A.N., Teregulova, A.N., and Safiullin, R.L., Mendeleev Commun., 2021, vol. 31, no. 2, p. 233.
Funding
This study was performed in accordance with the research plan at the Ufa Institute of Chemistry, Ufa Research Center, Russian Academy of Sciences (AAAA-A20-120012090019-1). All quantum-chemical calculations were performed on the equipment of the Chemistry Multiaccess Center of the Ufa Institute of Chemistry and Agidel Multiaccess Center, Ufa Research Center, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by L. Smolina
Abbreviations and notation: PES, potential energy surface; HPLC, high-performance liquid chromatography.
Rights and permissions
About this article
Cite this article
Safiullin, R.L., Teregulova, A.N., Yarullin, A.R. et al. para-Substituent Effect on the Decay Kinetics of the Isomeric Forms of Aromatic Nitroso Oxides. Kinet Catal 63, 172–179 (2022). https://doi.org/10.1134/S0023158422020082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158422020082