Abstract
The results of the working stability studies of cobalt catalysts based on SiO2 and Al2O3 promoted with Re and Al2O3 in the synthesis of hydrocarbons from CO and H2 in continuous tests for 200–300 h are presented. The prepared catalysts were characterized by transmission electron spectroscopy, temperature-programmed reduction with hydrogen, temperature-programmed desorption of CO, and X-ray fluorescence spectroscopy and tested at a temperature 200°C, a pressure of 0.1 MPa, and a GHSV of 100 h–1. It was determined that a cobalt–silica catalyst promoted with Al2O3 had the highest activity. It was established that the addition of Al2O3 to a cobalt–silica catalyst increased the conversion of CO and selectivity for C5+ hydrocarbons and inhibited the agglomeration of Co particles under the action of a reaction atmosphere in the Fischer–Tropsch synthesis. It was found that the initial conversion of CO increased by a factor of 2 upon the introduction of 0.1 wt % rhenium into the Co/γ-Al2O3 catalyst; however, the rate of its deactivation increased in this case due to an almost twofold increase in the size of cobalt particles in the course of synthesis after operation for 300 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The development of technologies for the production of synthetic hydrocarbons based on the Fischer–Tropsch (FT) synthesis is of considerable current interest primarily due to the need of converting nontraditional carbon-containing resources (associated petroleum gas, coal, biomass, etc.) and more stringent requirements imposed on the products obtained [1–3]. The FT synthesis is a heterogeneous catalytic process, which allows one to obtain a wide range of hydrocarbon products from CO and H2 depending on the catalyst used and synthesis conditions [4, 5]. Supported cobalt catalysts based on Al2O3, SiO2, and TiO2 are most widely used in industry [6–8]. With the use of supports based on Al2O3 and TiO2, a portion of cobalt forms difficult-to-reduce compounds with the support, which are inactive in the FT synthesis [9, 10]. It is possible to decrease the degree of metal–support interactions and increase selectivity for the formation of target products by the introduction of promoters—the noble metals Pt, Re, and Ru [16–18].
The stable operation of catalysts in a continuous mode is important for the industrial use of catalysts in order to produce C5+ hydrocarbons. Recently, a technology was developed and a pilot batch of cobalt–silica gel catalysts promoted with alumina was manufactured at ZAO Samara Catalyst Plant [19]. It was of interest to determine the stability of this catalyst and compare it with that of well-known samples.
Therefore, the aim of this work was to study the working stability of cobalt catalysts on various supports in the synthesis of hydrocarbons from CO and H2 in continuous long-term tests.
EXPERIMENTAL
Preparation of Hydrocarbon Synthesis Catalysts
The following four FT synthesis catalysts were prepared: Co/SiO2, Co–Al2O3/SiO, Co/γ-Al2O3, and Co–Re/γ-Al2O3.
The catalysts were prepared by the impregnation of porous supports. KSKG silica gel from OOO Salavat Catalyst Plant (GOST [State Standard] 3956-76) and AN γ-Al2O3 from OOO Novomichurinsk Catalyst Plant (TU [Technical Specifications] 2163-142-60 201 897-2010) were used as the supports.
An impregnation solution of cobalt nitrate (Co(NO3)2) with a concentration of 55% was used to prepare the catalysts. The support granules were immersed in the impregnation solution of cobalt nitrate and kept for 0.5 h at a temperature of 70–80°С. Rhenium was introduced into the catalysts by coimpregnation with cobalt nitrate and ammonium perrhenate (NH4ReO4). The concentration of ammonium perrhenate in the impregnation solution was maintained in such a way that the Re content of the prepared catalyst was 0.1 wt %. The procedure used for preparing the Co–Al2O3/SiO2 catalyst included the joint impregnation of the support with cobalt and aluminum nitrates [19].
After impregnation, an excess of the impregnation solution was removed, and the wet catalyst granules were subjected to heat treatment under the temperature-programmed conditions: 80°C, 4 h; 100°С, 1 h; 120°С, 1 h; 140°С, 1 h; and 400°С, 4 h; the heating rate was 10 K/min.
Catalyst Characterization Procedures
The cobalt content of the catalyst samples was determined by X-ray fluorescence analysis (XRF) on an ARLQUANT’X spectrometer (Thermo Scientific, Switzerland) under the following conditions: an atmosphere of air, a Teflon substrate, and an effective irradiation area of 48.9 mm2.
The freshly prepared catalysts were studied by temperature-programmed reduction with H2 (TPR-H2) using a ChemiSorb 2750 analyzer (Micromeritics, the United States) with a thermal conductivity detector (TCD). A sample of 0.1–0.15 g was placed in a quartz reactor arranged in a temperature-programmed furnace. Before the onset of TPR-H2, the catalyst sample was kept in a flow of He (20 mL/min) for 2 h at a temperature of 200°С to remove moisture and other adsorbed gases. Then, the sample was cooled to room temperature and the flow was switched to a mixture of 10% H2 and 90% N2 (20 mL/min). The reduction process was studied in a temperature range of 20–800°C with a heating rate of 5 K/min.
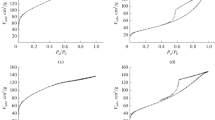
The catalysts were studied by the temperature-programmed desorption (TPD) of CO using a ChemiSorb 2750 analyzer (Micromeritics, the United States) with a thermal conductivity detector (TCD). A sample of about 0.1 g was placed in a U-shaped quartz reactor and kept in a flow of helium (20 mL/min) for 2 h at a temperature of 200°С to remove moisture and other adsorbed gases. Reduction with a hydrogen–nitrogen mixture (10% H2 and 90% N2) was carried out for 1 h at a temperature of 400°C. The pulse adsorption of CO was carried out at 20°С in a flow of helium (20 mL/min) until the surface of the sample was saturated. After saturation, the sample was purged with helium (20 mL/min) for 1 h at a temperature of 100°C to remove physically adsorbed gas. The desorption of CO was carried out by increasing the temperature from 100 to 800°C at a rate of 20 K/min.
The study of the catalysts by transmission electron microscopy (TEM) was carried out using a Tecnai G2 Spirit BioTWIN microscope (FEI, the Netherlands) with an accelerating voltage of 120 kV. A sample of the initial catalyst was preliminarily reduced with a nitrogen–hydrogen mixture (10% H2 + 90% N2) by linear heating from room temperature to 500°C for 1 h. The samples were ground in a flow of CO2 and applied to copper gauze in the form of suspensions (isopropyl alcohol–catalyst).
The particle size distribution of cobalt crystallites in prereduced catalysts was determined using transmission electron microscopy. The average crystallite size was calculated from the following formula [20]:
where ni is the number of particles with a diameter di.
The dispersity of cobalt (D, %) was calculated based on these data [21]:
where dav(Co0) is the average crystallite size of metallic cobalt, nm.
The standard deviation was determined from the formula [30]
Hydrocarbons were removed from the pores of catalysts after the synthesis (washed out) in a Soxhlet extractor. For this purpose, a Schott filter with a catalyst sample was placed in a reservoir located at the center of the extractor. 1,2-Dimethylbenzene (o-xylene) and n-heptane were used as solvents. The catalyst washing procedure included the extraction of hydrocarbons with o-xylene (2 h at a temperature of 144°C) and then extraction with n-heptane for 1 h at a temperature of 98°C.
The Fischer–Tropsch synthesis of hydrocarbons was carried out in a steel flow reactor (din = 16 mm) with a fixed catalyst bed (10 cm3) at atmospheric pressure (0.1 MPa), a GHSV of 100 h–1, and a constant temperature (T) of 200°C. The catalyst samples were preliminarily reduced in a flow of H2 at GHSV= 3000 h–1 and T = 400°С for 1 h. The catalysts were activated with synthesis gas by a stepwise increase in the temperature (2.5 K/h) from 150 to 200°С. After the catalyst activation, the synthesis was carried out in a continuous mode for 300 h.
The rate of catalysts deactivation was judged from the parameter RCD (%/h), which shows a decrease in the degree of conversion of CO after catalyst operation for 1 h. RCD was determined using the formula
where \(X_{{{\text{CO}}}}^{{\text{i}}}\) is the initial degree of conversion of CO, %; \(X_{{{\text{CO}}}}^{{\text{f}}}\) is the final degree of conversion of CO, %; and τ is time, h.
The synthesis gas and gaseous synthesis products were was analyzed by gas-adsorption chromatography on a Kristall 5000 chromatograph (Khromatek, Russia) with a thermal conductivity detector, a Hayesep R column (Khromatek, Russia), and a column with NaX molecular sieves. The former column was used for the analysis of С1–С5 hydrocarbons and СО2 (carrier gas, helium; flow rate, 15 mL/min), and the latter was used for the analysis of CO, Н2, and N2 (carrier gas, argon; flow rate, 15 mL/min) under temperature-programming conditions (80–240°C; heating rate, 8 K/min).
RESULTS AND DISCUSSION
Table 1 summarizes the characteristics of the prepared and spent catalysts. These data indicate that the cobalt content of the test catalysts varied in a range of 19.7–22.8%.
Figure 1 shows the TEM images and particle-size distribution diagrams of cobalt crystallites for reduced catalysts.
Cobalt particles 5–20 nm in size occurred on the surface of the Co/SiO2 catalyst (Fig. 1a); the average particle size was 14.8 nm (Table 1).
The addition of Al2O3 to the silica gel catalyst (Fig. 1b) significantly changed the dispersity of the active component: its value increased from 6.5 to 10.9%. The introduction of alumina facilitated a decrease in the cobalt particle size and narrowed the particle-size distribution. It is likely that an additive of 1.0 wt % Al2O3 inhibited the aggregation of cobalt metal nanoparticles, which manifested itself in the observed decrease in the average particle size. The distribution became almost unimodal; the average cobalt particle size was 8.8 nm, which is considered optimal for FT synthesis catalysts [22].
The particles of cobalt in the Co/γ-Al2O3 catalyst (Fig. 1c) had an average size of 21.8 nm (Table 1). The introduction of rhenium into the composition of Co/γ-Al2O3 (Fig. 1d) led to a significant decrease in the average size of cobalt nanoparticles from 21.8 to 13.9 nm (Table 1) and, as a consequence, to an increase in dispersity from 4.4 to 6.9%. The data on the effect of the addition of rhenium to the γ-Al2O3-based catalyst are consistent with published results [23–27].
Figure 2 and Table 2 show the TPR-H2 curves of catalyst samples and their characteristics, respectively.
The TPR-Н2 curves of all of the samples contained two intense reduction peaks (Fig. 2) due to the Co3+ → Co2+ and Co2+ → Co0 transitions in ranges of 262–324 and 337–583°С, respectively. A shift of peaks 1 and 2 to a range of higher temperatures was observed for the Co–Al2O3/SiO2 catalyst. This can be a consequence of interactions between the supported particles of cobalt oxide and Al2O3 [28].
The ratio S2/S1 between the areas of the two main peaks (Table 2) of SiO2-based catalysts, which indicates the amount of hydrogen consumed in the Co3+ → Co0 transition, is 3, which corresponds to a theoretically expected value based on the reaction stoichiometry of the stepwise reduction of cobalt oxide Co3O4 to Co0 [29].
The addition of rhenium to the Co/γ-Al2O3 catalyst led to a shift in the temperature maximum of peak 2 toward a lower temperature without a significant effect on the reduction temperature of cobalt oxide Co3O4 to CoО (peak 1). The experimental data on the effect of the rhenium promotion of γ-Al2O3-based catalysts on the reduction of CoO to Co0 are consistent with the conclusions of Tavasoli et al. [23] on the role of rhenium as a reduction promoter. Smaller S2/S1 area ratios for these catalysts may indicate that a portion of cobalt occurred in a difficult-to-reduce form.
The adsorption of CO on reduced catalysts was investigated using the TPD of CO. Figure 3 shows the TPD curves for CO from the reduced samples.
All of the catalyst samples exhibited pronounced profiles of the temperature-programmed desorption of CO. Two major desorption peaks were observed. The first low-temperature peak with a maximum at 207–220°C for catalysts based on SiO2 or 317–337°C for samples based on γ-Al2O3 can be attributed to the desorption of chemisorbed CO. The second high-temperature peak with a maximum at 672–772°C probably appeared as a result of the desorption of CO2 formed from chemisorbed CO by the Boudoir reaction [30]. The introduction of Al2O3 and Re promoters into the catalysts led to a significant increase in the amount of desorbed CO in the temperature range of the appearance of the first peak. Lapidus et al. [31] found that the first peak in the TPD of CO profile corresponds to the weakly bound adsorption of CO in a molecular form on the oxide catalyst surface. It characterizes bifunctional active surface centers involved in the process of polymerization.
Table 3 shows the average values of the main parameters of the catalytic activity of samples in the synthesis of hydrocarbons for 300 h of continuous operation.
All of the test catalysts were active in the synthesis of hydrocarbons from CO and H2. Under the experimental conditions, Co–Al2O3/SiO2 was most active: the conversion of CO was 78.6%, and selectivity and productivity for C5+ hydrocarbons were 64.2% and 11.3 kg m–3 h–1, respectively. Promotion with alumina led to a 10.8% increase in the catalyst productivity due to an increase in the conversion of CO and selectivity for C5+. In addition, the rate of catalyst deactivation decreased by 9.6% upon the introduction of alumina.
The introduction of rhenium into the composition of the Co/γ-Al2O3 catalyst increased the degree of conversion from 41.9 to 68.5%. At the same time, the selectivity for C5+ hydrocarbons decreased from 71.1 to 65.4% due to the more intense formation of gaseous reaction products C1–C4 and CO2. A significant increase in selectivity for CO2 (by a factor of 2.5) in the promoted sample may be associated with an increase in the rate of water gas shift reaction due to the facilitation of the dissociative adsorption of CO [32].
The rhenium promotion of the Co/γ-Al2O3 catalyst led to an increase in the rate of its deactivation by a factor of 2.9. Jacobs et al. [33] and Dalai and Davis [34] also noted that the introduction of noble metals into the composition of cobalt catalysts facilitated their accelerated deactivation.
Figure 4 shows the dependence of CO conversion and selectivity for CH4 on the duration of the synthesis. In general, all of the test catalysts demonstrated a regular decrease in the conversion of CO with time [35]. A decrease in activity was accompanied by a decrease in selectivity for C5+ hydrocarbons due to increased formation of methane and CO2.
An increase in selectivity for methane with time can be due to the accumulation of liquid C5+ hydrocarbons in the pores of the catalyst, which may lead to a decrease in the number of available active chain growth centers [32]. On the other hand, as noted by Iglesia et al. [36], the accumulation of synthesis products in the pores of the catalyst facilitated an increase in the molar ratio H2/CO inside a catalyst granule due to the different diffusion rates of the reactants. Indeed, Khasin [37] found that the diffusion coefficients of hydrogen and CO through a film of n-tetradecane at 200°С are 1.6 × 10–4 and 2.2 × 10–4 m2/s, respectively. This leads to an increase in the ratio Н2/СО on the catalyst surface and an increase in selectivity for methane formation [38].
The first 50 h of synthesis were characterized by the highest rate of decrease in the activity of all of the samples. It is likely that the active component of the catalyst was stabilized during this period under the influence of a reaction atmosphere.
The Co/SiO2 and Co–Al2O3/SiO2 catalysts exhibited similar initial conversions of CO and selectivity for the formation of C5+ hydrocarbons and CH4. However, after ~250 h of synthesis, a sharp decrease in activity was observed in the Co/SiO2 sample, which was accompanied by an increase in the fraction of methane formed and a decrease in the productivity of С5+ hydrocarbons.
A sharp increase in the degree of CO conversion from 48 to 90% was observed in the initial period upon the addition of rhenium to the γ-Al2O3-based catalysts (Fig. 4a). Vada et al. [39] found a similar effect and observed a rapid decrease in the activity of the Co–Re/γ-Al2O3 catalyst in comparison with that of a sample without rhenium.
The loss of catalyst activity is usually caused by a combination of factors that have different effects on the behavior of the catalytic system in the course of synthesis. The formation of a large amount of water at a high degree of CO conversion is considered a main reason for the deactivation of cobalt catalysts based on oxide supports. The effect of active metal oxidation under the action of Н2О leads to a decrease in the metal surface area of a cobalt catalyst [11]. In this case, the rate of oxidation of the active phase is directly proportional to the partial pressure of water [40].
For the TEM study of the catalysts after the synthesis, they were unloaded in a flow of nitrogen and subjected to two-stage washing with o-xylene and n-heptane in order to remove hydrocarbons from the pores. The TEM data (Fig. 5a, Table 1) showed that the dispersity and particle-size distribution of Co in the Co–Al2O3/SiO2 sample that worked for ~300 h were comparable with those of the initial reduced catalyst (Fig. 1b). It is likely that, in addition to an increase in the conversion of CO and the process selectivity for C5+ hydrocarbons, the introduction of Al2O3 into a cobalt–silica gel catalyst inhibited the aggregation of Co nanoparticles under the action of a reaction atmosphere in the FT synthesis conditions to facilitate stable catalyst operation.
The Co–Re/γ-Al2O3 catalyst after 300 h of operation exhibited a significant increase in the average particle size of Co (from 13.9 to 27.6 nm), which was a consequence of the agglomeration of cobalt particles under the action of a reaction atmosphere.
CONCLUSIONS
We studied the working stability of the supported cobalt catalysts in the synthesis of hydrocarbons from CO and H2 in continuous tests. It was determined that the cobalt–silica gel catalyst promoted by Al2O3 had the highest activity. The introduction of Al2O3 into Co/γ-Al2O3 increased the degree of CO conversion and selectivity for C5+ hydrocarbons and inhibited the aggregation of Co particles in the FT synthesis conditions under the action of a reaction atmosphere.
The addition of rhenium to the Co/γ-Al2O3 catalyst led to a significant increase (by a factor of ~2) in the conversion of CO in the initial period of catalyst operation (the first 50 h). However, the rate of deactivation on the promoted sample was almost three times higher, probably, due to a twofold increase in cobalt particle sizes under the action of the reaction atmosphere. Nevertheless, the conversion of CO even after 200 h of synthesis in the presence of Co–Re/γ-Al2O3 was higher by a factor of 1.5.
The addition of rhenium to the Co/γ-Al2O3 catalyst led to a significant increase (by a factor of ~2) in the conversion of CO in the initial period of catalyst operation (the first 50 h). However, the rate of deactivation on the promoted sample was almost three times higher, probably, due to a twofold increase in cobalt particle sizes under the action of the reaction atmosphere. Nevertheless, the conversion of CO even after 200 h of synthesis in the presence of Co–Re/γ-Al2O3 was higher by a factor of 1.5.
An increase in selectivity for the formation of methane in the course of synthesis was observed on all of the catalysts. This can be a consequence of the accumulation of liquid C5+ hydrocarbons in the catalyst pores, which leads to an increase in the molar ratio H2/CO inside a catalyst granule due to different rates of diffusion of the reactants.
As a result of this work, we determined that the rate of catalyst deactivation increased in the order Co/γ-Al2O3 < Co–Al2O3/SiO2 < Co/SiO2 < Co–Re/γ-Al2O3.
REFERENCES
Prieto, G., De Mello, M.I.S., Concepcion, P., Murciano, R., Pergher, S.B.C., and Martınez, A., ACS Catal., 2015, vol. 5, p. 3323.
Ellepola, J., Thijssen, N., Grievink, J., Baak, G., Avhale, A., and Schijndel, J., Comput. Chem. Eng., 2012, vol. 42, p. 2.
Krylova, A.Yu., Kulikova, M.V., and Lapidus, A.L., Solid Fuel Chem., 2014, vol. 48, p. 230.
Krylova, A.Yu., Solid Fuel Chem., 2014, vol. 48, p. 22.
Dry, M.E., Catal. Today, 2002, vol. 71, p. 227.
Kalechits, I.V., Khimicheskie veshchestva iz uglya (Chemical Substances from Coal), Moscow: Khimiya, 1980.
Lapidus, A.L., Krylova, A.Yu., Mikhailova, Ya.V., Sineva, L.V., and Erofeev, A.B., Solid Fuel Chem., 2011, vol. 45, p.71.
Krylova, A.Yu., Kinet. Catal., 2012, vol. 53, no. 6, p. 790.
Bessell, S., Appl. Catal., A, 1993, vol. 96, p. 253.
Jung, J.S., Lee, J.S., Choi, G., Ramesh, S., and Moon, D.J., Fuel, 2015, vol. 149, p. 118.
Ma, W., Jacobs, G., Keogh, R.A., Bukur, D.B., and Davis, B.H., Appl. Catal., A, 2012, vols. 437–438, p. 1.
Jacobs, G., Ma, W., Gao, P., Todic, B., Bhatelia, T., Bukur, D.B., Khalid, S., and Davis, B.H., Top. Catal., 2012, vol. 55, p. 811.
Ghasvareh, P. and Smith, K.J., Energy Fuels, 2016, vol. 30, p. 9721.
Merino, D., Perez-Miqueo, I., Sanz, O., and Montes, M., Top. Catal., 2015, vol. 59, p. 207.
Bor, Ø., Eri, S., Blekkan, E.A., Storsæter, S., Wigum, H., Rytter, E., and Holmen, A., J. Catal., 2007, vol. 248, p. 89.
Xiong, J., Borg, O., Blekkan, E.A., and Holmen, A., Catal. Commun., 2008, vol. 9, p. 2327.
Lapidus, A.L., Pavlova, V.A., Eliseyev, O.L., Gushchin, V.V., Davydov, P.E., and Chin, N.K., Pet. Chem., 2009, vol. 49, no. 4, p. 301.
Mikhailova, Ya.V., Sviderskii, S.A., Krylova, A.Yu., and Lapidus, A.L., Solid. Fuel. Chem., 2010, vol. 44, no. 5, p. 345.
Narochnyi, G.B., Yakovenko, R.E., Savost’yanov, A.P., and Bakun, V.G., Katal.Prom-sti., 2016, vol. 8, no. 2, p. 37.
Prieto, G., Martínez, A., Concepción, P., and Moreno-Tost, R., J. Catal., 2009, vol. 266, p. 129.
Wang, S., Yin, Q., Guo, J., Ru, B., and Zhu, L., Fuel, 2013, vol. 108, p. 597.
Borg, Ø., Dietzel, P.D.C., Spjelkavik, A.I., Tveten, E.Z., Walmsley, J.C., Diplas, S., Eri, S., Holmen, A., and Rytter, E., J. Catal., 2008, vol. 259, p. 161.
Tavasoli, A., Mortazavi, Y., Khodadadi, A.A., and Mousavian, M.A., Iran. J. Chem. Chem. Eng., 2005, vol. 24, p. 9.
Das, T.K., Jacobs, G., Patterson, P.M., Conner, W.A., Li, J., and Davis, B.H., Fuel, 2003, vol. 82, p. 805.
Borg, Ø., Hammer, N., Eri, S., Lindvag, O.A., Myrstad, R., Blekkan, E.A., Rønning, M., Rytter, E., and Holmen, A., Catal. Today, 2009, vol. 142, p. 70.
Borg, Ø., Eri, S., Blekkan, E.A., Storsæter, S., Wigum, H., Rytter, E., and Holmen, A., J. Catal., 2007, vol. 248, p. 89.
Tavasoli, A., Mortazavi, Y., Ali, K., Ali, A.A., Ali, M., and Ali, M., Iran. J. Chem. Chem. Eng., 2005, vol. 24, p. 9.
Zhang, Y., Nagamori, S., Hinchiranan, S., Vitidsant, T., and Tsubaki, N., Energy Fuels, 2006, vol. 20, p. 417.
Pardo-Tarifa, F., Cabrera, S., Sanchez-Dominguez, M., and Boutonnet, M., Int. J. Hydrogen Energy, 2017, vol. 42, p. 9754.
Fischer, N., Steen, E., and Claeys, M., J. Catal., 2013, vol. 299, p. 67.
Lapidus, A.L., Krylova, A.Yu., Mikhailova, Ya.V., Sineva, L.V., and Erofeev, A.B., Solid Fuel Chem., 2011, vol. 45, no. 2, p. 71.
Borg, Ø., Yu, Z., Chen, D., Blekkan, E.A., Rytter, E., and Holmen, A., Top. Catal., 2014, vol. 57, p. 491.
Jacobs, G., Patterson, P.M., Zhang, Y., Das, T., Li, J., and Davis, B.H., Appl. Catal., A, 2002, vol. 233, p. 215.
Dalai, A.K. and Davis, B.H., Appl. Catal., A, 2008, vol. 348, p. 1.
Zhou, W., Chen, J., Fang, K., and Sun, Y.H., Fuel Process. Technol., 2006, vol. 87, p. 609.
Iglesia, E., Soled, S.L., Fiato, R.A., and Via, G.H., J. Catal., 1993, vol. 143, p. 345.
Khasin, A.A., Gazokhimiya, 2008, no. 2, p. 28.
Botes, F.G., Niemantsverdriet, J.W., and Loosdrecht, J.A., Catal. Today, 2013, vol. 215, p. 112.
Vada, S., Hoff, A., Adnanes, E., Schanke, D., and Holmen, A., Top. Catal., 1995, vol. 2, p. 155.
Jacobs, G., Das, T.K., Patterson, P.M., Li, J., Sanchez, L., and Davis, B.H., Appl. Catal., A, 2003, vol. 247, p. 335.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation (state contract no. 10.2980.2017/4.6) and the President of the Russian Federation (grant no. MK-364.2019.3 for young scientists) and performed with the use of the equipment of the Nanotechnology Center of Collective Use at the Platov South-Russian State Polytechnic University (NPI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Abbreviations: FT, Fischer–Tropsch synthesis; TEM, transmission electron microscopy; TPR-H2, temperature-programmed reduction with hydrogen, TPD, temperature-programmed desorption; XRF, X-ray fluorescence analysis; and GHSV, gas hourly space velocity.
Rights and permissions
About this article
Cite this article
Yakovenko, R.E., Zubkov, I.N., Narochniy, G.B. et al. Effect of Re and Al2O3 Promotion on the Working Stability of Cobalt Catalysts for the Fischer–Tropsch Synthesis. Kinet Catal 61, 310–317 (2020). https://doi.org/10.1134/S0023158420020111
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420020111