Abstract
Recent advances in the synthesis of polymer-immobilized clusters and metal nanoparticles and their use in catalysis of many organic reactions were presented. The main types of polymer-immobilized catalysts were considered, including cluster-containing polymers, polymer-protected metal nanoparticles fixed on inorganic supports, and gel-immobilized catalysts. Special attention was paid to the role of the polymer matrix. It functions not only as a stabilizing agent that prevents the aggregation of nanoparticles and their leaching into the reaction medium, but also as a ligand in the coordination sphere of the metal site, which allows the advantages of homogeneous and heterogeneous catalysts to be combined in one catalyst.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
CONTENTS
Introduction
1. Cluster-containing polymers and their catalytic properties
2. Polymer-immobilized metal nanoparticles
3. Gel-immobilized catalysts
3.1 Catalysts based on metal nanoparticles immobilized in the hydrogel matrix
3.2. Catalysts based on macroporous cryogels with immobilized metal nanoparticles
4. Catalysts based on polymer-protected metal nanoparticles on inorganic supports
Conclusions
INTRODUCTION
Metal nanoparticles, also called nanoclusters or nanostructures, are the objects of active research due to their wide applicability in various fields such as catalysis, electronics, data recording and storage, sensors, and medicine [1–4]. The growing interest in the use of metal nanoparticles in catalysis is dictated by the following reasons. On the one hand, they have high specific surface area and are therefore as effective as homogeneous catalysts. On the other hand, they are characterized by high proportion of surface metal atoms that can play the role of potential active sites, which is significantly higher than in ordinary heterogeneous catalysts. The development of new catalytic systems combining the possibility of recycling the heterogeneous catalysts with a wide range of catalyzed reactions and high selectivity of homogeneous catalysts is a challenge today.
Nanocatalysis (in particular, metal nanoparticles encapsulated in polymers) as an actively developing area in modern catalysis is often considered as an intermediate field between homogeneous and heterogeneous catalysis [5–7]. In the case of polymer-immobilized metal complexes, the tendency toward combining the advantages of conventional homogeneous and heterogeneous catalysts and elimination of their disadvantages has already led to the creation of hybrid-phase catalytic systems [8–13]. They are characterized by the possibility of easy separation from the reaction mass and reuse like typical heterogeneous catalysts; high efficiency and better reproducibility characteristic of homogeneous catalysts, while the structure of heterogeneous catalysts substantially depends on the synthesis and processing procedure; lower sensitivity of their properties to oxygen and moisture traces due to the hydrophobic nature of the polymer matrix, etc. Multifunctional nanomaterials can also be obtained by designing complex hybrid nanostructures due to association of the metal core and the stabilizing shell of the organic ligand, polymer, ionic liquid, inorganic compound, support, etc. These associations and interactions are very important for fine control of the surface properties of the nanostructure and hence the catalytic efficiency of the hybrid nanocomposite. The role of the nonmetal component in such systems can be dual. On the one hand, the polymer matrix, e.g., stabilizes the highly dispersed, easily oxidizable metal nanoparticles, preventing their oxidation and leaching into the environment. On the other hand, it can act as a cocatalyst: its properties can be changed in a definite way for direct control over the reactivity and selectivity of the metal core. Thus, the size of metal nanoparticles obtained in the polyamidoamine matrix (PAMAM) depends on the number of functional groups in the dendrimer and its structure [14]. The number of internal amino groups determines the maximum number of metal ions that can be encapsulated in the polymer matrix for control over the nanoparticle size. In the cyclopropanation in the presence of a hybrid catalyst of dendrimer-encapsulated Au/SiO2 nanoparticles, the selectivity with respect to the cis-diastereoisomer increased fivefold compared to the homogeneous analog, which was explained by the steric effects of the dendrimer matrix [15].
Numerous experimental data and theoretical calculations indicate that the differences in the electronic and geometrical structures of supported individual atoms, metal clusters, and nanoparticles significantly affect the selectivity and activity of the catalytic process due to their size, nature of interaction with support and reagents, and the possibility of their evolution during the catalytic reaction [16]. For nanoparticles of less than 1 nm, over 90% of atoms are localized on the surface of the cluster, which can lead to a decrease in the coordination number, shortening of the metal–metal bond, easier oxidation, etc. These properties of subnanometer metal particles, as well as the high surface energies and surface/volume ratios, provide their exceptional catalytic activity and selectivity in many organic reactions. The Pd nanoclusters (with sizes of <2 nm) stabilized by the PEG–PNIPAM copolymer show very high activity in the Suzuki reaction of iodine- or chlorobenzene with phenylboronic acid (TOF = 4.3 × 104 h–1) [17]. The Cu30 nanoclusters in the matrix of the PAMAM–OH(G6) dendrimer matrix exhibit 100% selectivity in the hydrogenation of the C=O bond to the alcohol molecule in the presence of the C=C bond in the hydrogenated compound [18]. The presence of an induction period in some Au-catalyzed reactions, for example, in alkyne bromination and hydration with mononuclear compounds (HAuCl4 or AuCl) used as initial catalysts, is associated with in situ formation of catalytically active Au clusters [19]. The size-dependent catalytic properties of clusters in these reactions were confirmed in the case of specially synthesized Au5 and Au8 clusters encapsulated in the poly(aminoamide) (PAMAM) dendrimer molecule. Their activity was slightly lower than in the case of the in situ formed systems, in which the structures and coordination environments of the clusters were optimum.
In heterogeneous catalysis, the creation of monodisperse catalytic systems is a fundamental principle. The majority of catalysts used in chemical industry, energetics, and ecological systems are supported: active particles 1–100 nm in size are usually located on the inner surface of a porous support. This is explained by the fact that the properties of supported catalysts depend on the structure and size of the active phase; when its content decreases, a decrease in the particle size of the deposited metal leads to an increase in the number of active sites of the same composition involved in the catalytic process, which leads to an increase in the reaction rate and selectivity.
Significant progress has been achieved in the past decades in the use of polymer stabilizers for metal nanoparticles used as catalysts of numerous organic synthesis processes [20–26]. Along with stabilization of catalytic active sites, another effective strategy is catalyst separation and recycling. For example, the use of soluble polymers makes it possible to avoid diffusion limitations on the rate of the catalytic reaction. One of the recently developed approaches is based on the use of polymer supports with covalently bound molecules of ionic liquids, making use of some advantages of the latter such as effective stabilization and activation of catalysts, increased reaction rate and selectivity, and efficient recycling of catalytic systems. There are numerous examples of successful use of this strategy in immobilization of the catalytically active metal nanoparticles [27–30], including, e.g., chemoselective hydrogenation of unsaturated aldehydes and ketones by Pd nanoparticles stabilized by the phosphine-containing polymer-immobilized imidazolium ion (PdNP@PPh2-PIILP) and its PEG-modified derivative (PdNP@PPh2-PEGPIILP) [31] (Fig. 1, Table 1).
(a) Fragment of the structure of PdNPs@PPh2-PIILP and (b) diagram of the catalytic hydrogenation of cinnamaldehyde. The quantitative parameters of the reaction in the presence of polymer-immobilized Pd nanoparticles are given in Table 1.
Modification of support with PEG molecules increases both the catalyst efficiency in cinnamaldehyde conversion and the selectivity of C=C bond hydrogenation, which can reach 100%.
The same catalytic system with insignificant crosslinking (due to the presence of the tris-4-vinylphenylphosphine group) is highly efficient in the reduction of aromatic nitro compounds under moderate conditions and at low catalyst loads with TON = 3600 (TOF = 2580 h–1) and 274 000 (TOF = 17 125 h–1) for transfer hydrogenations and hydrations, respectively [32]. In addition, Pd-immobilized catalysts of this type are active in the Suzuki–Miyaura tandem cross-linking and reduction of nitroarenes with formation of biarylamines in high yields.
The goal of this work was to briefly discuss the synthetic strategy for obtaining polymer-immobilized clusters and metal nanoparticles, their structure, and catalytic properties in basic organic synthesis reactions.
1. CLUSTER-CONTAINING POLYMERS AND THEIR CATALYTIC PROPERTIES
Interest in transition and noble metal clusters is dictated not only by the wide variety of methods for ligand–metal binding and ligand configurations on the cluster surface, but also by the mobility of the metal frame itself [33, 34]. Due to the diversity of chemical composition and extremely interesting structures, polymer-immobilized clusters are potential components for the synthesis of various polymer materials with unusual properties. Cluster-containing polymers are of particular interest not only from the viewpoint of the fundamental aspects of composition and structure control and surface models of metal catalysts, but also as selective and highly efficient catalysts for many organic synthesis reactions. The general methods for the synthesis of cluster-containing polymers are based on physical or chemical encapsulation of cluster particles in polymers or (co)polymerization of cluster-containing monomers [35–39]. Also of interest are approaches that make it possible to include cluster-containing fragments in the main chain of a polymer molecule to form cluster-containing polymers, for example, {[{Pt3(μ-dppm)3}(μ-1,4-CNC6R4NC)][PF6]}n [40] and {Ru6(μ6-C)(CO)15(Ph2PC2PPh2)}n [41]), including bimetallic structures of the [−O(CH2)2(η-C5H4)-{Mo2Ir2(CO)10}(η-C5H4)(CH2)2OC(O)NHRNHC(O)−]n type [42]. To obtain cluster-containing monomers, two main approaches were developed: introduction of polymerizable ligands into polynuclear complexes (replacement of intrinsic ligands by analogs with multiple bonds, their oxidative addition, addition under mild conditions at M–M multiple bonds, etc.) or complementation of the corresponding ligands with clusters [36, 43, 44]. Carboxylates based on the Os3(CO)12 trinuclear carbonyl clusters and their Os3(CO)11(CH3CN) derivatives (μ-H)Os3(CO)10(μ-OR) (R = H, Ph), as well as the (μ-H)Os3(μ-OCNMe2)(CO)9PPH2CH2CH=CH2 cluster monomer, were synthesized in high yields [45–47]:

A number of sequential syntheses of Mo6-cluster carboxylates were based on the principle of outer-spheric substitution [48, 49]:
Subsequent (co)polymerization of metal-containing monomers of the given type leads to the formation of thermally stable cluster-containing polymers with controlled composition and sequence distribution in the polymer chain [50–53]. Special studies showed that copolymerization of cluster-containing monomers is preferable to modification of a functionalized polymer by cluster molecules [54]. The interaction of the Co2(CO)8 cluster with (co)polymers having dithiocarboxylate or thioamide functional groups was accompanied by the formation of side sulfur-containing organocobalt compounds or colloidal cobalt sulfide, which were difficult to separate from the desired product. At the same time, copolymers of MMA, styrene, or N-[tris-1,1,1(hydroxymethyl)methyl]acrylamide (number average polymer mass \({{\bar {M}}_{n}}\) = 6400–24 600) were synthesized with a quantitative yield, high purity, and strictly defined cluster fragments by copolymerization of monomeric alkylidinetricobalt nonacarbonyl or sulfidotricobalt heptacarbonyl clusters:

A multifunctional diblock copolymer containing a Co6Se8 molecular cluster was obtained by ring-opening metathesis polymerization of the cluster-containing monomer, which is a successful example of the synthesis of a linear copolymer based on chalcogenide clusters [55]. The integration of molecular clusters into block copolymers makes it possible to create highly organized assemblies from them for their use in catalysis. The numerous ligand binding sites on the cluster surface often lead to the formation of network structures; in some cases, soluble polymers can be obtained, as shown for dendrimer molecules based on Re6Se8 clusters [56]. Interestingly, the polymer-immobilized heterometallic clusters [ClOs3Au(CO)10(Ph3P] and [Co2Pt2(CO)8(Ph3P)2] were much more stable than their homogeneous or SiO2-supported analogs and showed high activity in olefin hydrogenations [57]. The mixed homogeneous [Co2Pt2(CO)8(Ph3P)2] metal cluster quickly transformed into the monometallic [Pt5(CO)6(PPh3)4] cluster. At the same time, the use of the polymer analogs of the (OC)9Co3CC(O)OCH2CH2OC(O)C(CH3)=CH2, (OC)9Co3CC6H4C(O)C(CH3)=CH2, and (OC)9Co3CC6H4CH=CH2 clusters as catalysts of hydroformylation showed that the active particles were the mono- or binuclear cobalt-carbonyl particles [58].
The catalytic activity of the polymer-immobilized Os3 clusters obtained by copolymerization of (CO)10Os3(μ-H)(N-4-vinylpyridine) [59] or [(μ‑H)Os3(μ-OCNMe2)(CO)9{P(CH2CH=CH2)Ph2}] [38] with styrene was studied in the oxidation of cyclohexene:

where CHHP is cyclohexenyl hydroperoxide, CHone is cyclohexenone, CHol is cyclohexenol, and ECH is epoxycyclohexene.
The kinetic curves of O2 absorption during the oxidation of cyclohexene are autocatalytic: the time within which the reaction rate becomes maximum is 40–108 min and is determined by the specific metal content (Table 2) [59]. It was shown that the autocatalytic nature of the process is due to the accumulation of hydroperoxide and effective generation of radicals by the reaction
with the parameters K = 1.86 L/mol and ki = 0.24 s–1 of the kinetic equation
The Os3 copolymers of the given type can easily be isolated from the reaction medium and used in repeated cycles without loss of activity, as shown in the case of the catalytic oxidative dehydrogenation of 2,3,6-trimethyl-1,4-hydroquinone (TMHQ) with formation of 2,3,6-trimethyl-1,4-quinone (TMQ), an intermediate in the synthesis of α-tocopherol (vitamin E) (Fig. 2, Table 3) [38].
(a) Fragment of the structure of the cluster-containing polymer [(μ-H)Os3(μ-OCNMe2)-(CO)9{P(CH2CH=CH2)Ph2}/styrene and (b) scheme of the catalytic oxidative dehydrogenation of TMHQ. The quantitative parameters of the reaction in the presence of polymer-immobilized Os3 nanoparticles are given in Table 3.
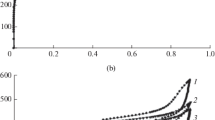
The efficiency of the catalytic action of polymer-immobilized Rh cluster complexes in hydrogenation was compared with that of conventional heterogeneous catalysts using Rh/C as an example (1% Rh), whose initial specific activity is 0.1 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{{\text{2}}}}}}}\)\({\text{g-atom}}_{{{\text{Rh}}}}^{{ - 1}}\,\,{{{\text{s}}}^{{ - 1}}}\) [60]. It was shown that the complexes under study were equal to the best heterogeneous catalysts in their activity (Fig. 3a). The initial rate of hydrogenation in the presence of the catalyst based on Rh6(CO)15CH3CN immobilized on the styrene–diphenylphosphoallyl copolymers with 4.16% Rh was low (0.02 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{{\text{2}}}}}}}\)\({\text{g-atom}}_{{{\text{Rh}}}}^{{ - 1}}\,\,{{{\text{s}}}^{{ - 1}}}\)); subsequently, as the number of cycles increased, the reaction rate increased to 0.4 \({\text{mo}}{{{\text{l}}}_{{{{{\text{H}}}_{{\text{2}}}}}}}\)\({\text{g-atom}}_{{{\text{Rh}}}}^{{ - 1}}\,\,{{{\text{s}}}^{{ - 1}}}\) (Fig. 3b).
(a) Hydrogen absorption curves during the hydrogenation of cyclohexene in the presence of the catalysts: (1) Rh/C, (2) copolymer of styrene with Rh6(CO)14(4-VPy)2, (3) Rh6(CO)15CH3CN immobilized on a styrene–diphenylphosphoallyl copolymer (4.16% Rh), and (4) copolymer of styrene with Rh6(CO)15(4-VPy). Conditions: Т = 40°С, \({{P}_{{{{{\text{H}}}_{{\text{2}}}}}}}\) = 1 atm, isopropanol solvent, 3.9 × 10−5 g-atom of Rh in the catalyst, 4.5 mmol of substrate. (b) Hydrogen absorption curves in five successive cycles of hydrogenation of cyclohexene in the presence of Rh6(CO)15CH3CN immobilized on the styrene–diphenylphosphoallyl copolymer (4.16% Rh). Conditions: Т = 40°С, \({{P}_{{{{{\text{H}}}_{{\text{2}}}}}}}\) = 1 atm, isopropanol solvent, 3.9 × 10−5 g-atom of Rh in the catalyst, 6.5 mmol of substrate. The curve number coincides with the cycle number.
The behavior of polymer-immobilized complexes in other reactions is similar [12] and associated with the “development” of the catalyst. It was assumed that cluster fragmentation occurs during hydrogenation, with highly active particles probably with higher nuclearity (nanosized particles) formed in low concentrations (true catalysts). These conclusions agree with the data of other studies. For example, it was shown that the activity of polymer-immobilized mononuclear Rh complexes increased with the degree of decarbonylation of Rh and formation of metal sites including \({\text{Rh}}_{n}^{0}\) [61]. Importantly, heterogenization of metal clusters allows one to isolate and study (e.g., by XP and IR spectroscopy) some of their catalytically active intermediates.
2. POLYMER-IMMOBILIZED METAL NANOPARTICLES
Immobilization of metal nanoparticles on the surface of solid supports is of interest not only from the viewpoint of studies of the relationship between the composition, structure, and properties of the metal nanoparticle–polymer catalyst system, nature of interaction, and specific role of macroligand in catalysis by heterogenized metal complexes and cluster particles, but also from the viewpoint of application of catalysts of this type. In practice, polymer supports of two main groups are used most often [12]. The first group are rigidly cross-linked microporous resins with a large specific surface area. The ligand groups of these resins are localized mainly on the surface and can contact with the components of the reaction medium. The second group consists of linear or weakly cross-linked reticular microporous polymers or their composites. They dissolve or swell in solvents; the catalytic active sites are located not only on the surface of the polymer, but also in its volume. The metal clusters can be immobilized due to the electrostatic attraction forces between the charged nanoclusters and the ionic groups of support via the formation of strong covalently bound or chelate complexes and during intermolecular interactions of various types [62].
Increased interest in metal nanoparticles stabilized with water-soluble polymers is dictated by the ability to control the growth kinetics and the nanoparticle size and distribution, which ultimately determines their catalytic, magnetic, electrical, optical, optoelectronic, biomedical, and other properties [63–69].
Among methods for the production of metal nanoparticles, the reduction of metal ions in water, organic solvents, and aqueous organic solutions in the presence of functional polymers and surfactants as reducers and stabilizers is widely used [70–76]. The metal ions adsorbed into multilayer polyelectrolytes, dendrimers, block copolymers, microgels, liposomes, polymer particles, and films or hydrogels that can be retained in solution due to the functional groups of the hydrophilic structure can easily be reduced to the corresponding metal nanoparticles, clusters, or metal oxides using suitable reducing agents such as NaBH4, AlBH4, H2, NH2NH2, citrate, etc. [77, 78].
The metal nanoparticles are stabilized with polymers in two different ways: physical (processes caused by van der Waals forces, dipole interactions, or weak, easily destroyed hydrogen bonds) or chemical adsorption [65]. The noncovalent interaction of nanoparticles with a macromolecule is rather weak (of the order of 10–4 J/m2); the efficiency of chemisorption is determined by the number of polar groups of the adsorbed polymer per unit surface regardless of the conformation of the macromolecules (expanded or globular). Importantly, the polymer should contain certain functional groups, which actively interact with the surface atoms of the nanoparticle, for example, as electron donors [71]. The efficiency of polymers as electron donors increases significantly if they contain centers of specific contacts acting simultaneously by the mechanism of charge and steric stabilization. Stable colloidal solutions of gold were synthesized in situ by the reduction of HAuCl4 in the presence of water-soluble polymers [79]. The most stable colloids were obtained with participation of polymers having hydrophobic ridges and hydrophilic side groups associated with gold ions.
The reduction of gold and silver was performed in one stage by boiling a mixture of HAuCl4 (or AgNO3) and polymers in the presence of KOH [80–87]. The following synthetic hydrophilic polymers were used as polymers that played the role of both stabilizers and reducing agents: poly-N-vinylpyrrolidone (PVPD), polyethylene glycol (PEG), polyethyleneimine (PEI), poly-N,N-dimethyl-N,N-diallylammonium chloride (PDMDAAC), poly-N-vinylbenzyl-N,N,N-trimethylammonium chloride (PVBTMAC), cationic polyelectrolyte JR-400, polyacrylic acid (PAA), natural polysaccharide gellan, and β-cyclodextrin (CD). The silver nanoparticles stabilized with PEG, PVPD, and CD were also obtained by the “dry” (mechanochemical activation) [88, 89] and “wet” (in ethanol, dimethylformamide, and dimethyl sulfoxide) methods [82]. The rate of formation of gold nanoparticles (NPs) during the heating in the presence of PEI, PDMDAAC, and CD was studied in [80]. It was found that in the presence of hydrophilic polymers, it changed in the sequence PDMDAAC > PEI > PVPD > JR-400.
There is no information in the literature on the effect of the molecular mass of the polymer on the size of NPs. Table 4 presents the results of measurements of the sizes of gold and silver NPs stabilized with PVPD of different molecular masses using dynamic laser light scattering (DLS) [82, 86]. According to the data of Table 4, the size of gold NPs increases with the molecular mass of the polymers.
The surface charge of gold NPs stabilized with PVPD is negative, –2.03 mV, while the surface charges of gold NPs stabilized with cationic polyelectrolytes (PEI and JR-400) are positive, +31.1 and +4.39 mV, respectively. The zeta potentials of silver NPs obtained in the presence of PVPD and CD are positive in DMSO and negative in DMF [82]. These results indicate that chemisorption of the nonionic and cationic type of macromolecules occurred on the surface of negatively charged gold NPs.
The Japanese scientists studied the colloidal catalysts [90–95]. When the Rh, Pd, Os, Ir, and Pt salts interacted in polyvinyl alcohol in water–methanol solutions, colloids with average Os, Ir, Pt, Rh, and Pd particle sizes of 1.0, 1.4, 2.7, 4.0, and 5.3 nm, respectively, formed [93]. The authors suggested a three-stage scheme for the formation of a colloidal dispersion: polymer coordination with metal ions, metal reduction with methanol to form small particles (for example, 0.8 nm for Rh) and their further enlargement (up to 4.0 nm). It was shown that Rh, Pd, Os, and Pt colloids are active and selective catalysts for hydrogenation of olefins at 30°C and atmospheric pressure of hydrogen. The PVPD-stabilized Au nanoclusters are effective catalysts for oxidations [96]. In the aerobic oxidative homo-coupling of potassium aryl trifluoroborate, the catalytic activity of Au/PVPD depends on the cluster size [97]. Au nanoclusters with an average diameter of dav = 1.3 ± 0.3 nm were highly active in homo-coupling, while the Au:PVP system (dav = 9.5 ± 1.0 nm) was catalytically inactive in the reaction under study. It is believed that the active sites of the catalyst are positively charged groups on the surface of the Au nanocluster, generated during the adsorption of molecular oxygen. High chemoselectivity in the hydrogenation of α,β-unsaturated aldehydes and ketones is exhibited by bimetallic Ag–Au/PVPD, which catalyze the reduction of the C=O bond more effectively than the addition at the C=C bond [98]. Optimization of the reaction according to the PVPD/metal ratio to ensure effective stabilization and according to the Ag/Au molar ratio, which allows a combination of the high chemoselectivity of Au action with Ag activity, makes it possible to significantly increase the selectivity of formation of allyl alcohols.
It is noteworthy that many studies were performed, especially in the last decade, on the synthesis of metal nanoparticles encapsulated in dendrimers that exhibited catalytic activity in various organic syntheses, including hydrogenation of numerous model compounds [99–107].
The interactions of solutions of linear polymers with transition metal salts can also give heterogeneous catalysts with a uniform distribution of spherical nanoparticles of 4–6 nm located throughout the polymer matrix (Fig. 4a). Such catalytic systems were studied in the hydrogenation of 2-propen-1-ol [108–111]. According to the XPS data, the reduction of the Pd complex with poly-2-vinylpyridine (P2VP) under the conditions of catalysis (in ethanol or water at 20–30°С) forms particles of a zero-valent metal [108].
The particles are enlarged after hydrogenation (Fig. 4b). However, closer examination of the reduced and spent catalysts shows that they are agglomerates of smaller starting NPs with sizes of 4–6 nm. It can be assumed that under conditions of liquid-phase hydrogenation, the polymer-metal complexes (PMCs) swell, increasing in size, and the metal particles encapsulated in them become available for activation of unsaturated organic compounds. After drying, the samples again shrink and are combined into larger aggregates due to the hydrophobic interactions of the polymer chain segments. Reuse of systems in catalysis does not lead to a decrease in their activity. Moreover, the reaction rate noticeably increases on the “developed” P2VP–Pd complex in some cases [108]. This is probably due to the fact that in the reaction medium, the PMC swells again, and the intermolecular hydrophobic interactions are weakened.
To reduce the effect of the diffusion factor, it seems promising to create the catalysts obtained by immobilization of the polymer–metal complexes on solid inorganic supports. In this case, both the technological parameters of heterogeneous catalysts (ease of separation from the reaction products and the possibility of regeneration and repeated use) and the activity inherent in homogeneous systems are preserved due to the formation of metal complexes with polymer ligands on the support surface, due to which the relative mobility active centers in the reaction medium is maintained.
Polyvinylpyrrolidone is actively used as a stabilizer of nanoparticles, including the case when they are deposited on oxides for the synthesis of catalysts [112–114]. The catalysts proposed by the authors were prepared by fixing the polymer-protected nanoparticles on a support, followed by the usual stages of high-temperature calcination and reduction, which led to a destruction of the polymer shell, with the metal nanoparticles preserved on the support. Thus, polymer-protected platinum nanoparticles were introduced by the capillary method into the mesoporous channels of the SBA-15 silica gel with a pore size of 9 nm [114]. Subsequent ultrasonic treatment allows localization of Pt nanoparticles mainly in the pores of silica gel (Fig. 5).
After the oxidizing and reducing treatment of the catalysts to remove the polymer stabilizer surrounding the nanoparticles, it is possible to form catalysts with different sizes of the active phase [112]. The resulting systems were studied in the hydrogenation of ethylene and cyclohexene. It was found that the particle sizes of the metals fixed on silica gel affect the selectivity of hydrogenation of cyclohexane and ethylene. However, the character of this dependence is rather complex and requires additional studies. At the same time, the authors believe are confident that using the developed approaches to the creation of catalysts, it is possible to regulate the sizes of nanoparticles fixed on a support and hence to selectively change their catalytic properties.
A similar approach was used to prepare catalysts based on dendrimer metal complexes, which were deposited on silica, calcinated, and reduced with hydrogen at high temperatures [100]. The dendrimer shell was burnt out, and a uniform layer of platinum nanoparticles formed on the support surface (Scheme 1):

Scheme 1 . Dendrimer template path of nanocatalyst formation.
The catalytic systems prepared in this way showed high activity in toluene hydrogenation and CO oxidation.
The polymer-immobilized Pd nanoparticles were actively studied in Suzuki–Miyaura cross-coupling reactions of aryl and vinylboronic acids with aryl or vinyl halides [115–118]. Palladium nanoparticles (d = 3.5 ± 1.5 nm) stabilized with poly(4-styrenesulfonic acid–co-maleic acid) (PSSA-co-MA) exhibit high catalytic activity in the reaction of p-bromoacetophenone or iodobenzene with phenylboronic acid with TOFs equal to 1980 and 5940 h–1, respectively [118]. Note that Pd/PSSA-co-MA and Ru/PSSA-co-MA synthesized in situ during the hydrolysis of ammonium borane (NH3BH3) are effective catalysts for the hydrolytic dehydrogenation of NH3BH3, which is considered as a promising material for hydrogen storage and production [117]. Similar properties are exhibited in this reaction by Ni nanoparticles stabilized with N-polyvinylpyrrolidone [119]. The polymer-immobilized catalyst showed good reproducibility without loss of activity in five cycles, which was explained by the absence of aggregation of metal particles due to the presence of a polymer matrix.
3. GEL-IMMOBILIZED CATALYSTS
In recent years, the joint efforts of chemists, physicists, and materials scientists have created various types of nanosized structures with metal nanoparticles in combination with functional polymers included in the building units. Nonionic, anionic, cationic, and amphoteric nano-, micro-, and macroporous hydrogels are promising polymer matrices for immobilization of metal nanoparticles, but they are still insufficiently studied [120]. The spatially cross-linked (three-dimensional) macromolecules containing various functional groups can change in size, shape, and morphology continuously or collapse when the external factors (temperature, pH of medium, ionic strength of solution, mixture of aqueous and organic solvents) are varied. This, in turn, allows subtle control of the structure, properties, and architecture of the nanoparticles immobilized in the hydrogel matrix. In addition, the hydrogel matrix can serve as some kind of a “microreactor” with which it is possible to perform exchange, redox, catalytic, and other types of reactions.
Recently, various types of polymer-immobilized nanocatalysts having high selectivity and stability of action have been obtained from hydro- and cryogels and noble metals. Immobilization of metal nanoparticles stabilized with water-soluble polymers in the matrix of hydro- and cryogels is of great interest from the viewpoint of creating the catalytic systems that simulate the action of metal enzymes and heterogenized homogeneous catalysts capable of accelerating the decomposition, hydrogenation, oxidation, isomerization, and other reactions.
The need to stabilize and immobilize metal nanoparticles in the hydrogel matrix is dictated by the fact that the nanoparticles having a large amount of excess surface energy can often be instantly passivated due to adsorption or coagulation.
3.1. Catalysts Based on the Metal Nanoparticles Immobilized in the Hydrogel Matrix
The hydrogels containing polymer-protected metal nanoparticles are complex systems with coiled linear macromolecules and metal nanoparticles lying in their pores and interstitial space. The linear macromolecules play the role of stabilizers that prevent the aggregation of active metal nanoparticles; the role of the hydrogel matrix is to limit the diffusion of the polymer-protected nanoparticles inside and outside the network.
The polymer-protected gold, silver, and palladium nanoparticles can be immobilized into the matrix of stimulus-sensitive hydrogels in two ways (Fig. 6) [121–130]. In the first case, the preliminarily dried polyacrylamide gel (PAAG) samples swollen in an aqueous solution of aurichlorohydric and tetrachloropalladic acid or silver nitrate are reduced with a sodium borohydride solution (Fig. 7).
In the second case, the monomer, crosslinking agent, and initiator previously dissolved in colloidal metal solutions are polymerized at 70°C for 30 min. The hydrogel samples containing the polymer-stabilized metal nanoparticles are homogeneous and have a characteristic color corresponding to the polymer-protected metal nanoparticles (Fig. 8). The average sizes of metal nanoparticles determined by SEM are presented in Table 5.
According to Table 5, the average sizes of metal nanoparticles stabilized with hydrophilic polymers are 10–60 nm, while the sizes of gold particles formed in the PAAG matrix by preliminary adsorption of HAuCl4 and subsequent reduction of NaBH4 are within 100 nm (Fig. 9). These data indicate that the metal nanoparticles stabilized with hydrophilic polymers have a narrower size distribution than those immobilized into the PAG matrix by adsorption and reduction.
SEM micrographs of the PAAG/PVPD-Pd0 sample [126].
The poly(N-isopropylacrylamide) hydrogel (PNIPAAMH) samples with polymer-protected palladium (PVPD–Pd0) immobilized in its matrix (PNIPAAMH/PVPD–Pd0) have a pronounced heat sensitivity in the range 25–40°С [131]. When the temperature rises to 40°С, the hydrogel is compressed and immobilized polymer-protected palladium PVPD–Pd0 is released into the medium; this is accompanied by turbidity of the aqueous solution surrounding the hydrogel (Fig. 10). As the temperature decreases to 25°C, the sample swells, and the aqueous solution surrounding the hydrogel becomes transparent due to the absorption of PVPD–Pd0 by the hydrogel matrix.
The catalytic activity of PNIPAAMH/PVPD–Pd0 was studied in the hydrogenation of 2-propen-1-ol [127]. As expected, the reaction rate increases sharply with temperature due to the exit of the catalytically active PVPD–Pd0 phase from the hydrogel matrix into the surrounding solution (Fig. 11). In contrast, a decrease in the temperature leads to a rapid decrease in the rate of substrate hydrogenation as a result of the absorption of PVPD–Pd0 by the hydrogel matrix. The sharp acceleration or deceleration of the catalytic reaction with a periodic change in temperature is explained by the fact that at T = 25°C, which is lower than the LCDT (lower critical dissolution temperature), PNIPAAMH is in an equilibrium-swollen state, and the exit of the PVPD–Pd0 catalyst to the surrounding solution is minimum (the net pores are closed). At T = 40°C, which is higher than the LCDT, PNIPAAMH is abruptly contracted (collapses), and the active sites cooperatively diffuse from the hydrogel into the surrounding solution (the net pores are open). Thus, a cyclic change in the temperature above or below the LCDT results in compression or swelling of PNIPAAMH (Fig. 12), which makes it possible to dose the homogeneous catalyst into the surrounding solution and thereby control the rate of the catalytic reaction.
The gel-immobilized gold NPs were studied in the decomposition of hydrogen peroxide and the oxidation of cyclohexane with hydrogen peroxide [129, 130]. The kinetics of the decomposition of hydrogen peroxide on gel-immobilized gold NPs obtained by three methods in the presence of various stabilizing polymers is shown in Fig. 13. The PEI-protected and gel-immobilized gold NPs obtained by sorption were most active in the decomposition of hydrogen peroxide (curve 1, Fig. 13). The effects of various factors (catalyst mass, reaction temperature, and substrate concentration) on the kinetics of hydrogen peroxide decomposition were studied on this catalyst.
A study of the oxidation of cyclohexane (CH) with hydrogen peroxide in the presence of gel-immobilized gold NPs showed rather high selectivity of this system for the formation of a mixture of cyclohexanol (CHol) and cyclohexanone (CHone) (Table 6) [130]. The conversion of cyclohexane did not exceed 7%.
Thus, the PEI-protected and gel-immobilized gold NPs obtained by sorption showed the highest catalytic activity in the decomposition of hydrogen peroxide and oxidation of cyclohexane with hydrogen peroxide. Note that, all other conditions being equal, the catalytic activity of gel-immobilized gold NPs is lower than that of NPs heterogenized by depositing the polymer-protected gold NPs on inorganic supports. This is probably due to the low stability of gel-immobilized NPs because of the destruction of the polymer net under the action of the hydrogen peroxide oxidant.
3.2. Catalysts Based on Macroporous Cryogels with Immobilized Metal Nanoparticles
Among the large number of polymer materials, macroporous cryogels occupy a special place. They are widely used in catalysis, biotechnology, biomedicine, bioseparation, and other fields of engineering and technology [132].
Systematic studies on the preparation of cryogel-immobilized metal nanoparticles and their use as catalytic systems were performed by the authors of [133–140]. These systems are regarded as some kind of microreactors.
The polyacrylamide (PAA) cryogel was modified with hydroxylamine according to Scheme 2 [140].

Scheme 2 . Сhemical modification of the PAA cryogel.
As a result, amido-PAA cryogel containing the functional groups of hydroxamic acids, which enable it to bind metal ions by the electrostatic mechanism, was prepared. After the reduction of metal ions (Co, Ni, Cu) with sodium borohydride in situ, copper, cobalt, and nickel nanoparticles immobilized in the cryogel matrix were obtained (Scheme 3).

Scheme 3 . In situ formation of copper nanoparticles in the matrix of the macroporous amido-PAA cryogel [140].
Subsequently, these nanocomposites were used as catalysts in the reduction of 2-nitrophenol (2-NPh). It was found that the composites based on copper nanoparticles had the highest catalytic activity.
The results of the use of a macroporous amphoteric cryogel based on methacrylic acid (MAA) and N,N-dimethylaminoethyl methacrylate (DMAEM) crosslinked with immobilized gold nanoparticles (DMAEM-MAA/GNPs) by methylene bisacrylamide (MBAA) as a catalyst of the reduction of 4‑nitrophenol (4-NPh) and a flow-type catalytic reactor (Fig. 14) were presented in [141–143]. It was shown that all cryogel nanosystems exhibited high catalytic activity at room temperature and atmospheric pressure [141]. Thus, the substrate conversion on nonionic, anionic, and cationic cryogel supports after hydrogenation of the tenth portion of 4-NPh was over 90%. The highest catalytic activity was shown by gold nanoparticles immobilized into the amphoteric cryogel matrix (Table 7).
The following thermodynamic parameters of hydrogenation of 4-NPh were obtained on the most active MAA-DMAEM/Au cryogel catalyst: Еа = 7.52 kJ mol–1, ΔHa = 4.91 kJ mol–1, and ΔSa = 284.34 J mol–1 K–1. The authors of [144–148] studied the hydrogenation of para-nitrobenzoic acid (PNBA) on the stationary phase of macroporous cryogel (DMAEM-MAA) containing gold and palladium nanoparticles at room temperature and atmospheric pressure.
When PNAA is hydrogenated on the DMAEM-MAA/Au catalyst, two by-products form in addition to para-aminobenzoic acid (PABA). One of them is para-azodibenzoate—the product of condensation of the nitroso compound with hydroxylamine. Its formation was confirmed by Raman spectroscopy, whose results are in satisfactory agreement with the data of [149] on the photoreduction of PNBA with silver nanoparticles (Scheme 4).

Scheme 4 . Mechanism of formation of para-azodibenzoate according to [148].
Another product of the conversion of PNAA is sodium 4-(4-aminobenzamido)benzoate, which results from the condensation of the terminal carboxyl and amine groups of PNAA catalyzed by gold nanoparticles (Scheme 5).

Scheme 5 . Mechanism of formation of sodium 4-(4-aminobenzamido)benzoate according to [149].
The condensation polymerization of the alkylated derivatives of PNAA, leading to the formation of alkylaminobenzamidobenzoates, was reported in [150].
Thus, the products of PNAA hydrogenation on the DMAEM-MAA/Au cryogel catalyst are its amino, azo, and amide derivatives.
In the case of PNBA hydrogenation on the DMAEM-MAA/Pd palladium-cryogel catalyst, there were no by-products. The conversion of PNAA into PABA was 40% at a ratio of [PNAA] : [NaBH4] = 1 : 50 mol/mol, 84% at [PNAA] : [NaBH4] = 1 : 100 mol/mol, and 100% for [PNAA] : [NaBH4] = 1 : 200 mol/mol.
4. CATALYSTS BASED ON POLYMER-PROTECTED METAL NANOPARTICLES ON INORGANIC SUPPORTS
The development of nanocatalysts obtained by immobilizing polymer-protected metal nanoparticles on solid inorganic supports (Fig. 15) is a promising area of research. When metal nanoparticles are deposited on inorganic supports, both the technological parameters of heterogeneous catalysts (ease of separation from reaction products, possibility of regeneration and reuse) and the activity of homogeneous systems are preserved due to the formation of metal nanoparticles on the support surface, which allows the relative mobility of active sites to be preserved in the reaction medium.
As is known [69, 151–153], on inorganic supports, in particular, on oxides, macromolecules are adsorbed almost irreversibly due to the cooperative interactions of the polymer chain segments with the support, preventing the leaching of the active phase, which is a solution to one of the most complex problems of supported complexes.
To increase the selectivity of hydrogenation of acetylene compounds, it was proposed that palladium-containing micellar polymer metal complexes be used as catalysts [154, 155]. The catalytic activity of micellar catalysts based on Pd and Pd–Au supported on a polystyrene-poly-4-vinylpyridine matrix and deposited on γ-Al2O3 in the hydrogenation of dehydrolinalool (2,6-dimethyloctaen-6-yn-1-ol-3) into linalool (2,6-dimethyloctadien-1,6-ol-3) was studied in [155]. The reaction performed in the diffuse region provides the highest selectivity of the process, but the data on the conditions of hydrogenation were not given.
E.M. Sulman et al. used the Pd/Al2O3 catalyst in the hydrogenation of complex acetylene alcohols with addition of KOH and pyridine [156, 157]. However, the catalyzed reactions often had low yields of the desired products because of their contamination with the modifying compounds such as pyridine, quinoline, metal salts, etc. Therefore, later this process was performed in the presence of palladium and rhodium catalysts modified with nitrogen-containing polymers on alumina [158–162]. In this case, high yields of olefin compounds were achieved. According to electron microscopy data, metal nanoparticles with sizes of 2–3 nm formed on the alumina surface.
A simple method of fixing a thin layer of PMC on the surface of inorganic sorbents (natural and synthetic zeolites, metal oxides) was proposed in [163]. The preparation of the catalysts involves sequential deposition (adsorption) of the polymer and then metal ions on the surface of the inorganic oxide.
A distinction of the method is excluded stages of high-temperature calcination and reduction of catalysts. During the hydrogenation of a wide range of unsaturated compounds [164–166] at temperatures of up to 40°С, these systems exhibit high activity, selectivity, and stability upon repeated use. The polymer-metal composite (complex) formed on the surface of the solid support retains its flexibility and mobility and is not washed out during catalysis because of the cooperative interactions of the functional groups of macromolecules with solid inorganic oxides. With an adequate choice of solvent, the swollen surface layer is a three-dimensional microreactor, with the catalytic process occurring inside this reactor and on its surface, as in the case of hydrogels.
The developed catalysts were tested in the hydrogenation of complex acetylene compounds such as 3,7,11-trimethyldodecyn-1-ol-3 (C15 acetylene alcohol), 3,7,11,15-tetramethylhexadecyn-1-ol-3 (C20 acetylene alcohol), 9-hexadecyn-1-ol, and 11-hexadecyn-1-ol [167, 168]. For example, the results of comparison of the conventional 1%Pd/ZnO catalyst prepared by the adsorption method with a PEG-containing sample in the hydrogenation of C20 acetylene alcohol also showed that the latter had significant advantages according to the three main parameters (activity, selectivity, and stability; Table 8). The rate of formation and the yield of 3,7,11,15-tetramethylhexadecen-1-ol-3 on the polymer-modified catalyst was higher than on the conventional Pd/ZnO system. The selectivity for the desired product on it increased by 11.2%, and TON increased threefold compared with that of the sample without PEG. The TOF of 670 h−1 on the polymer-containing catalyst indicates that the conversion of acetylene alcohol reached 86% within 5 min.
It was found that hydrogen is attached both at the triple and double bonds regardless of the nature of catalysts in C15 and C20 acetylene alcohols. During the reduction of 9- and 11-hexadecyn-1-ols, the process slowed down abruptly after the absorption of the first mole of hydrogen. The selectivity for the olefin derivative reached 99% on almost all the catalysts.
According to the XPS data, palladium in 1%Pd-PEG/ZnO is in the oxidized state (Pd2+) with a binding energy of Pd3d5/2 of ~337 eV (Fig. 16a). The treatment of the catalysts with hydrogen in a reactor at 40°C leads to a complete reduction of palladium to a zero-valence state. The Pd3d5/2 XPS spectrum contains a peak corresponding to the binding energy of palladium in the zero-valence state (~335.2 eV) (Fig. 16b). A similar picture was observed for the 1%Pd/ZnO catalyst.
The introduction of the polymer into the catalyst significantly affects the particle size of the active phase and their distribution over the support surface. In the 1%Pd/ZnO sample, metal particles with sizes of 30–50 nm form on zinc oxide (Fig. 17a). The palladium catalyst prepared with participation of PEG is characterized by the formation of uniformly localized small metal particles with sizes of 2–3 nm (Fig. 17b), which is clearly seen on micrographs with higher amplification (Fig. 17c). The particles are distributed uniformly probably due to the presence of a polymer film on the support surface, which prevents their aggregation. Thus, according to transmission electron microscopy, ZnO is represented by clearly faceted crystallites (Fig. 18a). When it is treated with polyethylene glycol, a transparent polymer layer forms on its surface (Fig. 18b).
Thus, the activity, selectivity, and stability of the PEG-modified Pd/ZnO catalyst are higher than those of the catalyst prepared without PEG. Polymer treatment has the greatest effect on stability (TON), which increases two- to fourfold.
The developed catalysts are actually heterogeneous and can easily be separated from the reaction products. Due to the flexibility, mobility, and swelling ability of the polymer chains of supported polymer–metal compounds, the relative mobility of active site is preserved in the reaction medium. The functions of the polymers are to form and fix metal nanoparticles on the support surface, prevent their agglomeration during catalysis, and increase the stability of active sites. The resulting catalysts showed high activity, selectivity, and stability in hydrogenations of complex acetylene alcohols to olefin derivatives under mild conditions.
Similar tendencies were found in the case of PVPD-stabilized gold nanocatalysts on aluminum and zinc oxides (Fig. 19) [81, 82, 85, 86]. The catalytic activity of polymer-stabilized gold and silver nanoparticles deposited on metal oxides in the decomposition of hydrogen peroxide was studied, and the optimum conditions were found: catalyst mass mcat = 30 mg, substrate concentration [H2O2] = 30%, and temperature T = 318 K (Fig. 20) [86]. The catalytic activity of polymer-stabilized gold nanoparticles deposited on zinc oxide in the oxidation of cyclohexane with hydrogen peroxide was reported in [169]. The results showed that on Au-PVPD/ZnO, cyclohexane is oxidized to cyclohexanol and cyclohexanone with higher conversion compared to that on Au-PAA/ZnO and Au-PEI/ZnO (Table 9).
Thus, polymer-protected gold nanoparticles heterogenized by fixing on the support surface are quite effective in the partial oxidation of cyclohexane under mild conditions. The activity and selectivity of the resulting catalysts depends on the nature of the polymer stabilizer and support; the size of noble metal nanoparticles depends on both the nature of the polymer stabilizer and its molecular mass.
A new approach to the creation of polymer-immobilized nanoparticles on the surface of an inorganic support by frontal polymerization of acrylamide complexes of noble metal nitrates in the presence of SiO2, Al2O3, C, ZnO, SnO2, etc. has been developed [165, 170–173]. The obtained polymer hybrid nanocomposites have a fairly developed surface and porous structure (Table 10), which ensures the accessibility of the active sites of the catalyst for reagents and their high activity in the reactions under study.
The PdAAm/SiO2 (Al2O3, C) complexes showed significant activity in the model hydrogenation of cyclohexene. The initial reaction rate in the presence of the poly-PdAAm/Al2O3 catalyst was almost three times higher than for the standard Pd/C under comparable conditions [165]. Importantly, the complexes retain their catalytic activity during recycling; the immobilized form makes it easy to isolate them from the reaction medium and reuse them. The polymer-immobilized Pd nanoparticles (poly-PdAAm/SiO2) exhibit not only efficiency, but also selectivity in the practically important hydrogenation of 2,4-dinitro- and 2,4,6-trinitrotoluenes—the rate of hydrogenation of the second nitro group is almost an order of magnitude lower than that of the first [170]. Due to the selective nature of the catalytic process, the desired products (i.e., the products of partial or complete hydrogenation) and, which is equally important, the catalytic intermediates can be isolated at appropriate stages. In addition to Pd0 (335.5 eV), the intermediate contains Pd atoms with a partially positive charge (Pdδ+, 337.0 eV), which facilitate the coordination of the substrate molecules; this affects the catalyst activity and, probably, the predominant hydrogenation of one nitro group. At the second stage, the binding energy in the Pd3d5/2 XPS spectrum shifts toward high energies (337.7 eV). The observed high selectivity of the hybrid nanocomposites is probably associated with steric factors, including those that are due to the presence of a polymer matrix, which leads to strong differentiation of the coordination of the substrate nitro groups.
A method for heterogenization of homogeneous Pt, Pd, Rh, Au, and Cu metal nanoparticles stabilized with dendrimers on mesoporous silica gel was suggested, which is based on successive reactions of complexation of metal ions with tertiary amino groups of PAMAM, reduction of metal ions with formation of nanoparticles, and fixation of the homogeneous catalyst on an inorganic support by impregnation [174–176]. The resulting heterogeneous catalysts were effective in the hydrogenation isomerization of methylcyclopentane. For example, along with significant hydrogenation activity at 200–225°C (TOF = 334 h–1), the Pt nanoclusters with sizes of 1.2–1.9 nm exhibit high selectivity (up to 99.6%) in isomerization of methylcyclopentane with ring opening at 94% conversion [175]. It can be assumed that the dendrimer molecules and the local environment of the catalytic sites created by them play a critical role in the nature of the catalytic effect of Pt nanoclusters. At the same time, the Pt nanoclusters (1.5 nm) stabilized with polyvinylpyrrolidone and fixed on the same silica gel (SBA-150) showed significantly lower activity and selectivity in the isomerization of methylcyclopentane (TOF = 19 h–1 at 280°C, 70% selectivity) [177].
To summarize, the above-considered approaches to the creation of polymer-protected metal nanoparticles fixed on inorganic supports make it possible to obtain systems possessing the advantages of both homogeneous (high activity and selectivity, mild process conditions) and heterogeneous catalysts (simple process for the preparation of the catalyst and its separation from the reaction products).
The method for the preparation of catalysts with nanosized particles of the active phase is simple and does not require large amounts of electricity at the calcination and reduction stages, which are necessary in the preparation of conventional supported catalyst systems for catalytic hydrogenation. The process is performed at room temperature using environmentally clean aqueous solutions. The preparation of catalysts includes the following stages: (1) strong adsorption of the soluble polymer on the support surface due to the cooperative interactions of segments of the polymer chains with the substrate and (2) addition of an active phase (e.g., palladium salt for hydrogenation). As a result, polymer-protected nanoparticles of the active phase form on the support surface.
Thus, treatment of the surface of conventional oxide substrates with polymers leads to their strong or irreversible adsorption. When introduced on this support, transition metal ions are localized around the functional groups of the polymer, forming nanosized particles of the active phase. The polymer “interlayer” leads to uniform distribution of the active phase and prevents its agglomeration, while the inorganic support facilitates the catalyst separation from the reaction products and makes the catalyst resistant against the action of the solvent.
5. CONCLUSIONS
Polymer-immobilized clusters and metal nanoparticles are of significant interest for catalysis due to the synthetic methodology developed in recent years and the possibility of controlled synthesis of required properties in a functional material. Immobilization of homogeneous metal-cluster nanoparticles on a solid surface without any loss of their catalytic activity is extremely important for practical applications of catalysts. Heterogenized polymer-immobilized metal nanoclusters are highly active catalysts for olefin hydrogenation [178]. The data on the electronic and geometrical structure of individual atoms, nanoclusters, and nanoparticles, including those on polymer supports, and their catalytic properties in various organic reactions, including CO oxidation, selective hydrogenation, and electro- and photocatalytic reactions, were summarized in a recent review [179]. Due to the moving geometrical structure of the polymer molecule, it is possible to overcome the steric limitations of conventional solid inorganic supports. In this case, e.g., a dendrimer polymer matrix with encapsulated metal nanoparticles can activate a heterogeneous catalyst, so that reactions usually occurring only with participation of homogeneous catalysts, for example, bond activation or aldol condensation start to occur in its presence. The dendrimer-encapsulated Au clusters exhibit lower catalytic activity than the nanoclusters formed in situ due to their optimized structure and coordination environment that is most favorable for catalysis [180]. Strict control of the size and shape of nanoparticles, including subnanosized ones, can be performed using amphiphilic (co)polymers and micelles based on them [181, 182]. This approach seems very promising for creating catalytic systems of diverse nanoarchitecture (spherical, rod-shaped, 3D-crosslinked). The Pd nanoclusters (average size ≈0.7 nm) stabilized with a polymer micelle of the amphiphilic copolymer of styrene 2-[(2-phenylallyloxy)methyl]oxirane and tetraethylene glycol-mono-2-phenyl-2 propenyl ether are highly active in hydrogenations and Heck reactions [181].
Currently, research in the field of hybrid-phase catalysts is under way. Hybrid catalysts such as the above-considered polymer-protected metal nanoparticles on inorganic oxide supports demonstrate improved performance (activity, selectivity, and service life) and the possibility of recycling. They also have additional functions such as microenvironment and configuration for various reaction routes and cascade reactions for products that are problematic to obtain in other ways. Also of interest are approaches to the development of highly stable catalytic mesostructures in which the polymer matrix that covers the surface of a metal nanostructure can be replaced with a metal oxide ligand acting as a cocatalyst [183]. These structures are expected to be effective in catalytic reactions with high activation energy. It is generally accepted that ligands significantly affect the conversion and selectivity of catalyzed reactions, as they do in the case of homogeneous catalysts. However, because of the large number of ligand types (polymers with different functional groups, including phosphorus-, sulfur-, nitrogen-containing ones) and various parameters, such as the chain length, configuration, steric hindrances, etc., general conclusions about their role require more detailed systematic studies.
Mixed metal clusters, including cluster-containing polymers, are unique molecular precursors for the preparation of bi- and multimetal catalysts. The spatial proximity of the metal sites provides multicenter interactions with the substrate, leading to the activation of molecules and thereby creating favorable conditions for increasing the catalytic activity. The binding of the substrate with M1 affects the interaction of this metal with M2, which, in turn, acts as a “ligand” for M1 and modifies its electronic interactions with the substrate [57]. The polymer-immobilized bimetallic nanostructures, such as Pt–Ru, Ru–Co, Pd–Ag, and Pt–Ni, are active in the reactions of hydrogen production by hydrolysis of aminoboranes [184], reduction of nitroaromatic compounds [185, 186], and oxidation of formaldehyde [187].
In recent years, another method appeared for heterogenizing homogeneous catalysts by introducing molecular complexes in the structure of metal-organic frameworks (MOFs) or coordination polymers; like zeolites, these catalysts are of considerable interest due to their unique porous crystal structure with a developed specific surface and a large internal pore volume [188–190]. The pores and channels of uniform size largely determine their catalytic selectivity, especially in the field of enantioselective catalysis [191]. Monocenter catalysts based on transition metals for hydrogenation of alkenes, imines, carbonyls, and heterocycles can be obtained and stabilized in the cavity of metal-framework structures [192, 193]. Moreover, the combination of synergistic and steric effects appearing after the introduction of Pt–SnOx in the cavity of the Zn-imidazole coordination polymer (ZIF-8) stimulated the design of a highly selective catalyst for hydrogenation of unsaturated aldehydes [194]. Other conceptual applications of MOFs to catalysis include frame stabilization of the active particle, combination of catalysis with chemical separation, post-synthetic incorporation of catalytic metal centers, and substrate-selective catalysis [195]. A recent review analyzed metal-organic framework structures as supports for immobilization of enzymes [196]. MOFs with a hierarchical structure can be regarded as multienzyme bioreactors for stepwise fixation of enzymes in suitable pores and channels.
Hybrid catalysts demonstrate better performance and stable activity, providing good reproducibility and scaling feature [197]. The design of the molecular architecture of the polymer matrix with encapsulated metal nanoparticles leads to high product selectivity, including diastereo- and enantioselectivity. However, combination of high activity and stability, 100% selectivity for the desired product, and possibility of recycling are still challenges that are waiting to be solved to find the optimum balance between these features. The development of modular catalytic systems with organic polymer components combined with inorganic nano- and subnanosized particles, whose catalytic properties can be finely tuned by changing various parameters, is currently a promising trend.
REFERENCES
Schmid, G., Nanoparticles. From Theory to Application, Weinheim: Wiley, 2004, p. 533.
Nanotechnology in Catalysis, Zhou, B., Han, S., Raja, R., and Somorjai, G., New York: Kluwer Academic/Plenum, 2003, p. 342.
Pomogailo, A.D. and Dzhardimalieva, G.I., Nanostructured Materials Preparation via Condensation Ways, Heidelberg: Springer, 2014, p. 460.
Uflyand, I.E. and Dzhardimalieva, G.I., Nanomaterials Preparation by Thermolysis of Metal Chelates, Cham: Springer, 2018, p. 549.
Gross, E., Toste, F. D., and Somorjai, G.A., Catal. Lett., 2015, vol. 145, p. 126.
Somorjai, G.A., Frei, H., and Park, J.Y., J. Am. Chem. Soc., 2009, vol. 131, p. 16589.
Philippot, K., Lignier, P., and Chaudret, B., Top. Organomet. Chem., 2014, vol. 48, p. 319.
Pomogailo, A.D., React. Polym., 1989, vol. 9, p. 109.
Savost’yanov, V.S., Pomogailo, A.D., and Ponomarev, A.N., Kinet. Katal., 1989, vol. 30, no. 6, p. 1414.
Pomogailo, A.D. and Uflyand, I.E., J. Mol. Catal., 1989, vol. 55, p. 429.
Dzhardimalieva, G.I. and Pomogailo, A.D., Kinet. Catal., 1998, vol. 39, no. 6, p. 821.
Pomogailo, A.D., Kinet. Catal., 2004, vol.45, no. 1, p. 61.
Pomogailo, A.D., Polym. Sci., Ser. A, 2008, vol. 50, no. 12, p. 1204.
Borodko, Y., Thompson, C.M., Huang, W., Yildiz, H.B., Frei, H., and Somorjai, G.A., J. Phys. Chem., p. 2011, vol. 115, p. 4757.
Johansson, M.J., Gorin, D.J., Staben, S.T., and Toste, F.D., J. Am. Chem. Soc., 2005, vol. 127, p. 18002.
Liu, L. and Corma, A., Chem. Rev., 2018, vol. 118, p. 4981.
Chen, Z., Liang, Y., Jiaa, D.S., Cui, Z.M., and Song, W.G., Chin. J. Catal., 2017, vol. 38, p. 651.
Maity, P., Yamazoe, S., and Tsukuda, T., ACS Catal., 2013, vol. 3, p. 182.
Oliver-Meseguer, J., Cabrero-Antonino, J. R., Dominguez, I., Leyva-Perez, A., and Corma, A., Science, 2012, vol. 338, p. 1452.
Crespo-Quesada, M., Andanson, J.-M., Yarulin, A., Lim, B., Xia, Y.N., and Kiwi-Minsker, L., Langmuir, 2011, vol. 27, p. 7909.
Zhao, Y., Baeza, J.A., Koteswara Rao, N., Calvo, L., Gilarranz, M.A., Li, Y.D., and Lefferts, L., J. Catal., 2014, vol. 318, p. 162.
Dong, Y., Jin, Y., Wang, J., and Zhang, M., Chem. Eng. J., 2017, vol. 324, p. 303.
Albuquerque, B.L., Denicourt-Nowicki, A., Mériadec, C., Domingos, J.B., and Roucoux, A., J. Catal., 2016, vol. 340, p. 144.
Evangelisti, C., Panziera, N., D’Alessio, A., Bertinetti, L., Botavina, M., and Vitulli, G., J. Catal., 2010, vol. 272, p. 246.
Chavda, N., Trivedi, A., Thakarda, J., Agrawal, Y.K., and Maity, P., Catal. Lett., 2016, vol. 146, p. 1331.
Dai, Y., Yu, P., Zhang, X., and Zhuo, R., J. Catal., 2016, vol. 337, p. 65.
Gruttadauria, M., Liotta, L.F., Salvo, A.M, C., Giacalone, F., La Parola, V., Aprile, C., and Noto, R., Adv. Synth. Catal., 2011, vol. 353, p. 2119.
Liu, X., Zhao, X., and Lu, M., Catal. Lett., 2015, vol. 145, p. 1549.
Burguete, M.I., García-Verdugo, E., Garcia-Villar, I., Gelat, F., Licence, P., and Luis, S.V., and Sans, V., J. Catal., 2010, vol. 269, p. 150.
Moreno-Marrodan, C., Barbaro, P., Catalano, M., and Taurino, A., Dalton Trans., 2012, vol. 41, p. 12666.
Doherty, S., Knight, J.G., Backhouse, T., Abood, E., Alshaikh, H., Fairlamb, I.J.S, Bourne, R.A., Chamberlain, T.W., and Stones, R., Green Chem., 2017, vol. 19, p. 1635.
Doherty, S., Knight, J.G., Backhouse, T., Bradford, A., Saunders, F., Bourne, R.A., Chamberlain, T.W., Stones, R., Clayton, A., and Lovelock, K., Catal. Sci. Technol., 2018, vol. 8, p. 1454.
Gonzalez-Moraga, G., Cluster Chemistry, Berlin: Springer, 1993, p. 304.
Gubin, S.P., Khimiya klasterov. Osnovy klassifikatsii i stroenie (Chemistry of Clusters. Basics of Classification and Structure), Moscow: Nauka, 1987.
Bar-Sela, G. and Warchawsky, A., React. Polym., 1983, vol. 1, p. 149.
Pomogailo, A.D. and Savost’yanov, V.S., Metallosoderzhashchie Monomery i polimery (Metal-Containing Monomers and Polymers), Moscow: Khimiya, 1988, p. 384.
Kubeil, M., Stephan, H., Pietzsch, H.-J., Geipel, G., Appelhans, D., Voit, B., Hoffmann, J., Brutschy, B., Mironov, Y.V., Brylev, K.A., and Fedorov, V.E., Chem. Asian J., 2010, vol. 5, p. 2507.
Pomogailo, S.I., Ershova, V.A., Shilov, G.V., Virovets, A.V., Pogrebnyak, V.M., Podberezskaya, N.V., Golovin, A.V., Dzhardimalieva, G.I., and Pomogailo, A.D., J. Organomet. Chem., 2005, vol. 690, p. 4258.
Efremova, O.A., Brylev, K.A., Kozlova, O., White, M.S., Shestopalov, M.A., Kitamura, N., Mironov, Y.V., Bauer, S., and Sutherland, A.J., J. Mater. Chem. C, 2014, vol. 2, p. 8630.
Bradford, A.M., Kristof, E., Rashidi, M., Yang, D.-S., Payne, N.C., and Puddephatt, R.J., Inorg. Chem., 1994, vol. 33, p. 2355.
Johnson, B.F.G., Sanderson, K.M., Shephard, D.S., Ozkaya, D., Zhou, W., Ahmed, H., Thomas, M.D.R., Gladden, L., and Mantle, M., Chem. Commun., 2000, p. 1317.
Lucas, N. T., Humphrey, M. G., and David Rae, A., Macromolecules, 2001, vol. 34, p. 6188.
Pomogailo, A.D., Platinum Met. Rev., 1994, vol. 38, no. 2, p. 60.
Pomogailo, A.D., Russ. Chem. Rev., 1997, vol. 66, no. 8, p. 679.
Ershova, V.A., Golovin, A.V., Shcludyakova, L.A., Semyannikov, P.P., Pomogailo, S.I., and Pomogailo, A.D., Russ. Chem. Bull., 2000, no. 8, p. 1448.
Maksakov, V.A., Kirin, V.P., Konchenko, S.N., Bravaya, N.M., Pomogailo, A.D., Virovets, A.V., Podberezskaya, N.V., Baranovskaya, I.G., and Tkachev, S.V., Russ. Chem. Bull., 1993, p. 1236.
Bravaya, N.M. and Pomogailo, A.D., in Metal-Containing Polymeric Materials, Pittman, C.U., Jr., Carraher, C.E., Jr., Zeldin, M., and Culberston, B., Eds., New York: Plenum Publ. Corp., 1996, p. 51.
Golubeva, N.D., Adamenko, O.A., Boiko, G.N., Petrova, L.A., Olkhov, Yu.A., and Pomogailo, A.D., Inorg. Mater., 2004, vol. 40, no. 3, p. 306.
Adamenko, O.A., Lukova, G.V., Golubeva, N.D., Smirnov, V.A., Boiko, G.N., Pomogailo, A.D., and Uflyand, I.E., Dokl. Phys. Chem., 2001, vol. 381, no. 3, p. 275.
Bravaya, N.M., Pomogailo, A.D., Maksakov, V.A., Kirin, V.P., Belov, G.P., and Solovieva, T.I., Russ. Chem. Bull., 1994, no. 3, p. 381.
Bravaya, N.M., Pomogailo, A.D., Maksakov, V.A., Kirin, V.P., Grachev, V.P., and Kuzaev, A.I., Russ. Chem. Bull., 1995, no. 6, p. 1062.
Wong, W.-T., Wong, W.-Y., and Yip, C.-W., J. Cluster Sci., 1995, vol. 6, no. 2, p. 311.
Liang, W., Li, D., Lau, P.-L., Xu, L., Yang, C., Wei, M., Chan, W.T.-K., and Wong, W.-Y., J. Organomet. Chem., 2018, vol. 870, p. 8.
Gressier, J.C., Levesque, G., and Patin, H., Polym. Bull., 1982, vol. 8, p. 55.
Voevodin, A., Campos, L. M., and Roy, X., J. Am. Chem. Soc., 2018, vol. 140, no. 16, p. 5607.
Wang, R. and Zheng, Z., J. Am. Chem. Soc., 1999, vol. 121, p. 3549.
Buchwalter, P., Rose, J., and Braunstein, P., Chem. Rev., 2014, vol. 115, no. 1, p. 28.
Withers, H.P. and Seyferth, D., Inorg. Chem., 1983, vol. 22, p. 2931.
Kholuiskaya, S.N., Pomogailo, A.D., Bravaya, N.M., Pomogailo, S.I., and Maksakov, V.A., Kinet. Catal., 2003, vol. 44, no. 6, p. 761.
Pomogailo, S.I., Dorokhov, V.G., Dzhardimalieva, G.I., Pomogailo, A.D., Lyakhovich, A.M., and Mikhailova, S.S., Kinet. Catal., 2006, vol. 47, no. 5, p. 719.
Bhaduri, S. and Khwaja, H., J. Chem. Soc., Dalton Trans., 1983, no. 2, p. 419.
Pomogailo, A.D. and Dzhardimalieva, G.I., Metallopolimernye gibridnye nanokompozity (Metal-Polymer Hybrid Nanocomposites), Moscow: Nauka, 2015.
Hodge, P. and Sherrington, D. Polymer-Supported Reactions in Organic Synthesis, New York: Wiley, 1980, p. 484.
Bekturov, E.A. and Kudaibergenov, S.E., Kataliz polimerami (Catalysis by Polymers), Alma-Ata: Nauka, 1988, p. 182.
Pomogailo, A.D., Polimernye immobilizovannye metallokompleksnye katalizatory (Polymer Immobilized Metal Complex Catalysts), Moscow: Nauka, 1988, p. 303.
Bekturov, E.A. and Kudaibergenov, S.E., Catalysis by Polymers, Heidelberg: Huthig and Wepf Verlag Zug., 1996, p. 153.
Bekturov, E.A., Kudaibergenov, S.E., Zharmagambetova, A.K., Iskakov, R.M., Ibraeva, Zh.E., and Shmakov, S.N., Polimer-protektirovannye nanochastitsy metallov (Polymer-Protected Nanoparticles of Metals), Almaty: Institute of Organic Catalysis and Electrochemistry after D. V. Sokol’sky, 2010, p. 274.
Ibraeva, Zh.E., Kudaibergenov, S.E., and Bekturov, E.A., Stabilizatsiya nanochastits metallov gidrofil’nymi polimerami (Stabilization of Nanoparticles with Hydrophylic Polymers), Saarbrücken: Lambert Academic Publishing, 2013, p. 376.
Pomogailo, A.D., Rozenberg, A.S, and Uflyand, I.E., Nanochastitsy metallov v polimerakh (Nanoparticles of Metals in Polymers), Moscow: Khimiya, 2000, p. 672.
Ibraeva, Zh.E., Zharmagambetova, A.K., Ketts, I., Bekturov, E.A., and Kudaibergenov, S.E., Khim. Zh. Kazakhstana, 2008, vol. 1, no. 19, p. 245.
Zakarina, N.A. and Bekturov, E.A., Izv. NAN RK, Ser. Khim., 2008, no. 1, p. 3.
Bergbreiter, D.E., Case, B.L., Liu, Y-S., and Caraway, J.W., Macromolecules, 1998, vol. 31, p. 6053.
Mohan, Y.M., Lee, K., Premkumar, T., and Geckeler, K.E., Polymer, 2007, vol. 48, p. 158.
François, N.J., Allo, S., Jacobo, S.E., and Daraio, M.E., J. Appl. Polym. Sci., 2007, vol. 105, p. 647.
Metal Clusters in Catalysis, Gates, B.C., Guezi, L., and Knosinger, H., Eds., Amsterdam: Elsevier, 1986, p. 234.
Shan, J. and Tenhu, H., Chem. Commun., 2007, vol. 44, p. 4580.
Wang, Y., Yan, R., Zhang, J.Z., and Zhang, W.Q., J. Mol. Catal. A: Chem., 2010, vol. 317, p. 81.
Park, S., Murthy, P.S.K., Park, S., Mohan, Y.M., and Koh, W.G., J. Ind. Eng. Chem., 2011, vol. 17, p. 293.
Mayer, A.B.R. and Mark, J.E., Eur. Polym. J., 1998, vol. 34, p. 103.
Kudaibergenov, S.E., Tatykhanova, G.S., and Baigaziyeva, E., Proc. Int. Conf. Nanomaterials: Applications and Properties, NAP-2012, Alushta, Crimea, 2012, vol. 1, p. 3.
Yesmurzayeva, N., Selenova, B., and Kudaibergenov, S., J. Am. Nanomaterials, 2013, vol. 1, p. 1.
Kudaibergenov, S.E., Baigaziyeva, E.K., Yesmurzayeva, N.N., Nurakhmetova, Zh.A., Selenova, B.S., Proc. Int. Conference. Nanomaterials: Applications and Properties, Crimea, September 16–21, 2013, vol. 2, no. 2, p. 02PCN03.
Baygazieva, E.K., Yesmurzayeva, N.N., Tatykhanova, G.S., Mun, G.A., Khutoryanskiy, V.V., and Kudaibergenov, S.E., Int. J. Biol. Chem., 2014, vol. 7, no. 1, p. 14.
Kudaibergenov, S.E. and Tatykhanova, G.S., Int. J. Biol. Chem., 2014, vol. 6, no. 2, p. 40.
Ibrayeva, Zh., Baigaziyeva, E., Yesmurzayeva, N., Tatykhanova, G., Yashkarova, M., and Kudaibergenov, S., Macromol. Symp., 2015, vol. 351, p. 51.
Yesmurzayeva, N.N., Selenova, B.S., and Kudaibergenov, S.E., Supramol. Catal., 2015, vol. 2, no. 2, p. 1.
Nurgaziyeva, E.K., Tatykhanova, G.S., Mun, G.A., Khutoryanskiy, V.V., and Kudaibergenov, S.E., Proc. Int. Conf. Nanomaterials: Applications and Properties, 2015, vol. 4, p. 42.
Inventor’s Certificate no. 69560, 2011.
Tatykhanova, G., Mukazhanova, Zh., Baigazieva, E., Yashkarova, M., Orazzhanova, L., Abdullin, Kh., and Kudaibergenov, S., Proc. Int. Conf. Nanomaterials: Applications and Properties (NAP-2011), 2011, vol. 1, part 1, p. 165.
Hirai, H., Ohtaki, M., and Komiyama, M., Chem. Lett., 1987, p. 149.
Toshima, N., Macromol. Symp., 2000, vol. 156, p. 45.
Toshima, N., J. Macromol. Sci. Chem., 1990, vol. 27, nos. 9–11, p. 1225.
Hirai, H., Nakao, Y., Toshima, N., J. Macromol. Sci., Chem., 1979, vol. A13, no. 6, p. 727.
Hirai, H., J. Macromol. Sci., Chem., 1979, vol. A13, no. 5, p. 633.
Toshima, N. and Wang, Y., Langmuir, 1994, vol. 10, p. 4574.
Tsunoyama, H., Ichikuni, N., and Tsukuda, T., Langmuir, 2008, vol. 24, p. 11327.
Sakurai, H., Tsunoyama, H., and Tsukuda, T., J. Organomet. Chem., 2007, vol. 692, p. 368.
Mertens, P.G.N., Vandezande, P., Ye, X., Poelman, H., Vankelecom, I.F.J., and De Vos, D.E., Appl. Catal., A, 2009, vol. 355, p. 176.
Karakhanov, E., Maximov, A., Zolotukhina, A., Mamadli, A., Vutolkina, A., and Ivanov, A., Catalysts, 2017, vol. 7, p. 12.
Lang, H., May, R.A., Iversen, B.L., and Chandler, B.D., J. Am. Chem. Soc., 2003, vol. 125, no. 48, p. 14832.
Scott, R.W.J., Wilson, O.M., and Crooks, R.M., J. Phys. Chem. B, 2005, vol. 109, no. 2, p. 692.
Zhang, W., Li, L., Du, Y., Wang, X., and Yang, P., Catal. Lett., 2009, vol. 127, nos. 3–4, p. 429.
Ornelas, C., Aranzaes, J.R., Salmon, L., and Astruc, D., Chem. Eur. J., 2008, vol. 14, p. 50.
Ornelas, C., Ruiz Aranzaes, J., Cloutet, E., Alves, S., and Astruc, D., Angew. Chem., 2007, vol. 119, p. 890.
Deraedt, C., Salmon, L., and Astruc, D., Adv. Synth. Catal., 2014, vol. 356, p. 2525.
Peng, X., Pan, Q., and Rempel, G.L., Chem. Soc. Rev., 2008, vol. 37, p. 1619.
Jin, Z., Xiao, H., Zhou, W., Zhang, D., and Peng, X., R. Soc. Open Sci., 2017, vol. 4, p. 1714.
Zharmagambetova, A.K., Golodov, V.A., and Saltykov, Yu.P., J. Mol. Catal., 1989, vol. 55, p. 406.
Zharmagambetova, A.K., Mukhamedzhanova, S.G., and Bekturov, E.A., React. Polym., 1994, vol. 24, p. 17.
Zharmagambetova, A.K., Mukhamedzhanova, S.G., and Dusenbina, B., React. Polym., 1994, vol. 24, p. 21.
Zharmagambetova, A.K., Mukhamedzhanova, S.G., and Bekturov, E.A., in Polimernye elektrolity, gidrogeli, kompleksy i katalizatory (Polymer Electrolites, Hydrogels, Complexes and Catalysts), Almaty: Institute of Organic Catalysis and Electrochemistry after D.V. Sokol’sky, 2007, p. 224.
Somorjai, G.A., Contreras, A.M., Montano, M., and Rioux, R. M., Proc. Natl. Acad. Sci. U. S. A., 2006, vol. 103, no. 28, p. 10577.
Hoefelmeyer, J.D., Niesz, K., Somorjai, G.A., and Don Tilley, T., Nano Lett., 2005, vol. 5, no. 3, p. 435.
Rioux, R.M., Song, H., Hoefelmeyer, J. D., Yang, P., and Somorjai, G.A., J. Phys. Chem. B, 2005, vol. 109, no. 6, p. 2192.
Li, Y., Hong, X.M., Collard, D.M., and El-Sayed, M.A., Org. Lett., 2000, vol. 2, p. 2385.
Narrayanan, R. and El-Sayed, M.A., J. Am. Chem. Soc., 2003, vol. 125, p. 8340.
Metin, O., Sahin, S., and Ozkar, S., Int. J. Hydrogen Energy, 2009, vol. 34, p. 6304.
Metin, O., Durap, F., Aydemir, M., and Ozkar, S., J. Mol. Catal. A: Chem., 2011, vol. 337, p. 39.
Umegaki, T., Yan, J.-M., Zhang, X.-B., Shioyama, H., Kuriyama, N., and Xu, Q., Int. J. Hydrogen Energy, 2009, vol. 34, p. 3816.
Kudaibergenov, S., Nueraje, N., and Khutoryanskiy, V., Soft Matter, 2012, vol. 8, p. 9302.
Kudaibergenov, S.E., Dolya, N., Tatykhanova, G., Ibraeva, Zh.E., Musabaeva, B.Kh., Yashkarova, M.G., and Bimendina, L.A., Eurasian Chem.-Technol. J., 2007, vol. 9, no. 3, p. 177.
Dolya, N., Musabaeva, B.Kh., Yashkarova, M.G., Bimendina, L.A., and Kudaibergenov, S.E., Vestn. NAN RK, 2007, no. 2, p. 20.
Dolya, N.A., Yashkarova, M.G., Musabaeva, B.Kh., Zharmagambetova, A.K., and Kudaibergenov, S.E., Izv. Nauch.-Tekh. Obshchestva “Kakhak”, 2007, no. 17, p. 231.
Svetlichnyi, D.S., Dolya, N., Govenko, P., Musabaeva, B.Kh., Yashkarova, M.G., and Kudaibergenov, S.E., Eurasian Chem.-Technol. J., 2007, no. 10, p. 41.
Dolya, N.A., Svetlichnyi, D.S., Kaliaskarova, B.A., Musabaeva, B.Kh., Yashkarova, M.G., Zharmagambetova, A.K., Ketts, I., and Kudaibergenov, S.E., Dokl. NAN RK, 2008, no. 3, p. 67.
Kudaibergenov, S. E., Ibraeva, Zh. E., Dolya, N. A., Musabaeva, B.Kh., Zharmagambetova, A.K., and Koetz, J., Macromol. Symp., 2008, vol. 274, p. 11.
Dolya, N.A., Zharmagambetova, A.K., Musabaeva, B.Kh., and Kudaibergenov, S.E., Vestn. NAN RK, 2008, no. 1, p. 55.
Dolya, N.A., Musabaeva, B.Kh., Yashkarova, M.G., and Kudaibergenov, S.E., Khim. Zhurn. Kazakhstana. Spets. Vyp., 2008, no. 21, p. 139.
Kudaibergenov, S.E. and Tatykhanova, G.S., Int. J. Biol. Chem., 2014, vol. 6, no. 2, p. 40.
Kudaibergenov, S.E., Tatykhanova, G.S., and Selenova, B.S., J. Inorg. Organomet. Polym. Mater., 2016, p. 1.
Dolya, N. and Kudaibergenov, S., Temperature-Responsive Polymers: Chemistry, Properties and Applications, Khutoryanskiy, V., Ed., London: Wiley, 2018, p. 357.
Okay, O. and Lozinsky, V.I., Adv. Polym. Sci., 2014, vol. 263, p. 103.
Sahiner, N. and Seven, F., Energy, 2014, vol. 71, p. 170.
Sahiner, N. and Yildiz, S., Fuel Process. Technol., 2014, vol. 126, p. 324.
Sahiner, N., Seven, F., and Al-lohedan, H., Water Air and Soil Pollution, 2015, vol. 226, no. 4, p. 10.
Ajmal, M., Demirci, S., Siddiq, M., Aktas, N., and Sahiner, N., Colloids Surf., A, 2015, vol. 486, p. 29.
Sahiner, N., Yildiz, S., and Al-Lohedan, H., Appl. Catal., B, 2015, vol. 166, p. 145.
Sahiner, N. and Yasar, A. O., Fuel Process. Technol., 2016, vol. 144, p. 124.
Yildiz, S., Sahiner, M., and Sahiner, N., Eur. Polym. J., 2015, vol. 70, p. 66.
Demirci, S. and Sahiner, N., Water Air and Soil Pollution, 2015, vol. 226, no. 3, p. 64.
Klivenko, A.N., Tatykhanova, G.S., Nuraje, N., and Kudaibergenov, S., Bull. Karaganda Univ. Ser. Chem., 2015, vol. 80, no. 4, p. 10.
Tatykhanova, G.S., Klivenko, A.N., Kudaibergenova, G.M., and Kudaibergenov, S.E., Macromol. Symp., 2016, vol. 363, no. 1, p. 49.
Kudaibergenov, S., Proc. IX Int. Bermzhan Symp. on Chemistry and Technology, Almaty, December 9–10, 2016, p. 21.
Klivenko, A.N., Yergazieva, E., and Kudaibergenov, S.E., Proc. Int. Conf. Nanomaterials: Application & Properties (NAP), 2016, p. 02NSA03-1-02NSA03-5. https://doi.org/10.1109/NAP.2016.7757304
Kudaibergenov, S., Aldabergenov, M., Dauletbekova, M., Kabdrakhmanova, S., Ibrayeva, Zh., and Selenova, B., Proc. World Congress of Engineers and Scientists WSEC-2017, Astana, 2017, vol. 3, p. 32.
Aldabergenov, M., Dauletbekova, M., Toleutay, G., Klivenko, A., and Kudaibergenov, S., Proc. 7th International Conference Nanomaterials: Application and Properties (NAP-2017), Zatoka, Odessa, September 10–15, 2017, p. 03NNSA27-1-03NNSA27-3. https://doi.org/10.1109/NAP.2017.8190273
Aldabergenov, M., Dauletbekova, M., Shakhvorostov, A., Toleutay, G., Klivenko, A., and Kudaibergenov, S., J. Chem. Technol. Metall., 2018, vol. 53, no. 1, p. 17.
Kudaibergenov, S., Dauletbekova, M., Toleutay, G., Kabdrakhmanova, S., Seilkhanov, T., and Abdullin, Kh., J. Inorg. Organomet. Polym., 2018, vol. 28, p. 2427.
Muniz-Miranda, M., J. Anal. Bioanal. Tech., 2015, vol. 6, p. 1.
Shibasaki, Y., Abe, Y., Sato, N., Fujimori, A., and Oishi, Y., Polym. J., 2010, vol. 42, p. 72.
Lipatov, Yu.S. and Sergeeva, L.M., Adsorbtsiya polimerov (Adsorption of Polymers), Kiev: Naukova dumka, 1972, p. 195.
Parfitt, C.D. and Rochester, C.H., Adsorption From Solution at the Solid/Liquid Interface, London: Academic press, 1983.
Lipatov, Yu.S., Mezhfaznye yavleniya v polimerakh (Interphase Phenomena in Polymers), Kiev: Naukova dumka, 1980, p. 260.
Usanov, A.E., Demidenko, G.N., Mikhailov, I.A., and Chernyshov, D.M., in Proc. 12th Intern. Conf. of Young Scientists on Chemistry and Chemical Technology MKKhT-98, Moscow, 1998, p. 4.
Usanov, A.E., Semagina, N.V., Demidenko, G.N., and Mikhailov, I.A., i in Proc. 12th Intern. Conf. on High-Tech Chemical Technology, Yaroslavl, 1998, p. 190
Sannikov, O.B., Popov, O.S., Sul’man, E.M., Ankudinova, T.V., and Shakhova, M.K., Voprosy Kinetiki i kataliza: Mezhvuzovskii sbornik, Ivanovo Institute of Chemical Technology, 1980, p. 108.
Sul’man, E.M., Popov, O.S., and Samokhvalov, G.I., Kataliz i katal. protsessy proizvodstva khimiko-farmokologicheskikh preparatov (Catalysis and Catalytic Processes for the Production of Chemical and Pharmacological Preparations), Moscow, 1985, vol. 2, p. 93.
Sidorov, A.I., Sul’man, E.M., Bronshtein, L.M., Ankudinova, G.V., Avtushenko, Yu.E., Mirzoeva, E.Sh., Baukova, E.Yu., and Valetskii, P.M., Kinet. Katal., 1993, vol. 34, no. 1, p. 87.
Mirzoeva, E.Sh., Bronstein, L.M., Valetsky, P.M., and Sulman, E.M., React. Polym., 1995, vol. 24, p. 243.
Sulman, E., Bodrova, Y., Matveeva, V., Semagina, N., Cerveny, L., Kurtc, V., Bronstein, L., Platonova, O., and Valetsky, P., Appl. Catal., A, 1999, vol. 76, no. 1, p. 75.
Bronstein, L., Chernyshov, D., Volkov, I., Ezernitskaya, M., Valetsky, P., Matveeva, V., and Sulman, E., J. Catal., 2000, vol. 196, no. 2, p. 302.
Semagina, N, Joannet, E, Parra, S., Sulman, E., Renken, A., and Kiwi-Minsker, L., App. Catal., A, 2005, vol. 280, p. 141.
RK Patent 22029, 2009.
Zharmagambetova, A.K., Polimer-protektirovannye nanochastitsy metallov (Polymer Protected Nanoparticles of Metals), Almaty: Nauka, 2010, p. 231
Golubeva, N.D., Dyusenalin, B.K., Selenova, B.S., Pomogailo, A.D., Zharmagambetova, A.K., and Dzhardimalieva, G.I., Kinet. Catal., 2011, vol. 52, no. 2, p. 242.
Zharmagambetova, A.K., Seitkalieva, K.S., Talgatov, E.T., Auezkhanova, A.S., Dzhardimalieva, G.I., and Pomogailo, A.D., Kinet. Catal., 2016, vol. 57, no. 3, p. 360.
Zharmagambetova, A.K., Zamanbekova, A.T., Mukhamedzhanova, S.G., and Tumabaev, N.Zh., Izv. NAN RK, Ser. Khim., 2009, no. 4, p. 61.
Zharmagambetova, A.K., Zamanbekova, A.T., Darmenbaeva, A.S., Auezkhanova, A.S., Dzhumekeeva, A.I., and Talgatov, E.T., Teor. Eksp. Khim., 2017, vol. 53, no. 4, p. 250.
Esmurzaeva, N.N., Baigazieva, E.K., Nurakhmetova, Zh.A., Erzhanova, D.S., Selenova, B.S., and Kudaibergenov, S.E., Proc. of Russian Congress on Catalysis, Samara, October 2–5, 2014, p. 55.
Dzhardimalieva, G.I., Dorokhov, V.G., Golubeva, N.D., Pomogailo, S.I., Lyakhovich, A.M., Savchenko, V.I., and Pomogailo, A.D., Russ. Chem. Bull., 2009, no. 10, p. 2070.
Pomogailo, A.D. and Dzhardimalieva, G.I., J. Catal., 2013, p. 12. https://doi.org/10.1155/2013/276210
Kalinina, K.S., Golubeva, N.D., Dzhardimalieva, G.I., and Pomogailo, A.D., Macromol. Symp., 2015, vol. 351, no. 1, p. 81.
Pomogailo, A.D., Kalinina, K.S., Golubeva, N.D., Dzhardimalieva, G.I., Pomogailo, C.I., Knerel’man, E.I., Protasova, S.G., and Ionov, A.M., Kinet. Catal., 2015, vol. 56, no. 5, p. 694.
Huang, W., Kuhn, J. N., Tsung, C. K., Zhang, Y., Habas, S.E., Yang, P., and Somorjai, G.A., Nano Lett., 2008, vol. 8, p. 2027.
Deraedt, C., Melaet, G., Ralston, W.T., Ye, R., and Somorjai, G.A., Nano Lett., 2017, vol. 17, p. 1853.
Ye, R., Yuan, B., Zhao, B., Ralston, W.T., Wu, C.-Y., Unel Barin, E., Toste, D.F., and Somorjai, G.A., J. Am. Chem. Soc., 2016, vol. 138, p. 8533.
Alayoglu, S., Aliaga, C., Sprung, C., and Somorjai, G.A., Catal. Lett., 2011, vol. 141, p. 914.
Toshima, N., Shiraishi, Y., and Teranishi, T., J. Mol. Catal. A: Chem., 2001, vol. 177, p. 139.
Liu, L. and Corma, A., Chem. Rev., 2018, vol. 118, p. 4981.
Oliver-Meseguer, J., Leyva-Perez, A., Al-Resayes, S.I., and Corma, A., Chem. Commun., 2013, vol. 49, p. 7782.
Okamoto, K., Akiyama, R., Yoshida, H., Yoshida, T., and Kobayashi, S., J. Am. Chem. Soc., 2005, vol. 127, p. 2125.
Menezes, W.G., Zielasek, V., Dzhardimalieva, G.I., Pomogailo, S.I., Thiel, K., Wohrle, D., Hartwig, A., and Baumer, M., Nanoscale, 2012, vol. 4, p. 1658.
An, K., Alayoglu, S., Musselwhilte, N., Plamthottam, G., Malaet, G., Lindeman, A.E., and Somorjai, G.A., J. Am. Chem. Soc., 2013, vol.135, p. 16689.
Rakap, M., Appl. Catal., A, 2014, vol. 478, p. 15.
Udumula, V., Tyler, J.H., Davis, D.A., Wang, H., Linford, M.R., Minson, P.S., and Michaelis, D.J., ACS Catal., 2015, vol. 5, p. 3457.
Ranjith, K.S., Celebioglu, A., and Uyar, T., Nanotechnology, 2018, vol. 29, p. 245602. https://doi.org/10.1088/1361-6528/aab9da
He, F.-G., Du, B., Sharma, G., and Stadler, F.J., Polymers, 2019, vol. 11, p. 674.
Wang, Z., Chen, G., and Ding, K., Chem. Rev., 2008, vol. 109, p. 322.
Corma, A., Garcia, H., and Xamena, F.X.L., Chem. Rev., 2010, vol. 110, p. 4606.
Chizallet, C., Lazare, S., Bazer-Bachi, D., Bonnier, F., Lecocq, V., Soyer, E., Quoineaud, A., and Bats, N., J. Am. Chem. Soc., 2010, vol. 132, p. 12365.
Ma, L., Abney, C., and Lin, W., Chem. Soc. Rev., 2009, vol. 38, p. 1248.
Ji, P., Manna, K., Lin, Z., Urban, A., Greene, F.X., Lan, G., and Lin, W., J. Am. Chem. Soc., 2016, vol. 138, p. 12234.
Cohen, S.M., Zhang, Z., and Boissonnault, J.A., Inorg. Chem., 2016, vol. 55, p. 7281.
Lan, X., Xue, K., and Wang, T., J. Catal., 2019, vol. 372, p. 49.
Lee, J.Y., Farha, O.K., Roberts, J., Scheidt, K.A., Nguyen, S.T., and Hupp, J.T., Chem. Soc. Rev., 2009, vol. 38, p. 1450.
Drout, R.J., Robison, L., and Farha, O.K., Coord. Chem. Rev., 2019, vol. 381, p. 151.
Kudaibergenov, S., Gels, 2019, vol. 5, p. 1.
Funding
This study was performed by G.I.D. under the government contract (state registration no. АААА-А19-119041090087-4).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Abbreviations and notation: DLS, dynamic light scattering; DEAEMA, N,N-diethylaminoethyl methacrylate; MAA, methacrylic acid; MBAA, methylene-bis-acrylamide; LCDT, lower critical dissolution temperature; 4-NPh, 4-nitrophenol; P2VP, poly-2-vinylpyridine; PАM, polyacrylamide; PAAG, polyacrylamide gel; PABA, para-aminobenzoic acid; PAA, polyacrylic acid; PVBТTMAC, poly-N-vinylbenzyl-N,N,N-trimethylammonium chloride; PVPD, poly-N-vinylpyrrolidone; PDMDAAC, poly-N,N-dimethyl-N,N-diallylammonium chloride; PMCs, polymer-metal complexes; PNBA, para-nitrobenzoic acid; PNIPAM, poly-N-isopropyl acrylamide; PNIPAMG, poly-N-isopropyl acrylamide gel; PEG, polyethylene glycol; PEI, polyethylene imine; XPS, X-ray photoelectron spectroscopy; SEM, scanning electron microscopy; CH, cyclohexane; cyclohexene; CD, β-cyclodextrin; JR-400, cationic polyelectrolyte; NP, nanoparticle; PAMAM, poly(aminoamide); PIIL, polymer-immobilized ionic liquid; PSSA-co-MA, poly(4-styrenesulfonic acid-со-maleic acid); TMQ, 2,3,6-trimethyl-1,4-quinone; TOF, turnover frequency, the maximum number of moles of the product that formed per unit time on 1 mol of the catalyst, time−1;TON, turnover number, the number of moles of the product (molecules of the product) that formed on 1 mol (one site) until the catalyst completely lost its activity.
Rights and permissions
About this article
Cite this article
Dzhardimalieva, G.I., Zharmagambetova, A.K., Kudaibergenov, S.E. et al. Polymer-Immobilized Clusters and Metal Nanoparticles in Catalysis. Kinet Catal 61, 198–223 (2020). https://doi.org/10.1134/S0023158420020044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0023158420020044