Abstract
The review summarizes the results obtained by the authors in a study of the polymerization of 1‑hexene on a supported titanium–magnesium catalyst. The effects of the composition of a complex titanium–magnesium catalytic system, the composition of a reaction medium, and the conditions of polymerization on the activity of the catalyst and the molecular weight, molecular-weight distribution, and isotacticity of the resulting polyhexene were considered. The main factors (the composition of a catalytic system and the polymerization conditions) that allow one to control the catalyst activity and the molecular structure of polyhexene over wide ranges were determined. Specific features of the polymerization of 1-hexene in comparison with the polymerization of ethylene and propylene on titanium–magnesium catalysts are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Polyhexene (PH) is a promising polymer material for use in various fields of technology with consideration for the possibilities of regulating its molecular weight and structure over wide ranges by the use of catalysts of various types and compositions. Thus, ultra-high-molecular-weight polyhexene is used as advanced drag reducing additives in the transportation of oil and oil products through pipelines. PH has good vibration-absorbing properties, and it can be used to obtain polymer composite materials with damping properties. As an elastomeric polymer, it can be used to prepare polymer compositions with polydiene elastomers possessing high adhesive properties.
Catalytic systems of various compositions can be used for the synthesis of polyhexene; they include Ziegler–Natta titanium–magnesium catalysts the modern versions of which are widely used in the production of polyethylene and polypropylene. Studies on the polymerization of 1-hexene on various types of titanium–magnesium catalysts were reported [1–9]. In particular, data on the activity of various types of titanium–magnesium catalysts, which for the most part have low activity, and the molecular-weight characteristics of polyhexenes obtained under various polymerization conditions were published. However, these results do not allow us to identify the most important factors responsible for the effects of catalytic system composition and polymerization conditions on the activity of the catalysts and the molecular structure of the polymer, which are necessary for the target-oriented regulation of the activity of catalysts and the molecular-weight characteristics and isotacticity of polyhexene.
In 2014–2019, we studied the kinetics of 1-hexene polymerization on a highly active supported titanium–magnesium catalyst (TMC), which is commonly used for the stereospecific polymerization of propylene [10–13]. As a result, we determined the effect of the composition of a complex TMC-based catalytic system on the rate of polymerization and the molecular-weight characteristics and isotacticity of the resulting polyhexene and studied the effect of polymerization conditions (the reaction temperature and the composition of a reaction medium) on the rate of polymerization and the molecular-weight characteristics of polyhexene. Based on the experimental data, we established the role of individual chain transfer reactions, which are responsible for the molecular weight of polyhexene, and calculated the kinetic characteristics of these reactions.
This review summarizes the results that we obtained in the study of the polymerization of 1-hexene on a highly active supported titanium–magnesium catalyst and published previously [10–13]. The review consists of two sections. The first section considers the effect of the composition of the catalytic system and the composition of a reaction medium on the activity of the catalyst and the molecular-weight characteristics of the resulting polyhexene. Kinetic data obtained by studying the influence of 1-hexene polymerization conditions on the rate of polymerization and the molecular weight of the resulting polymer are discussed in the second section.
RESULTS AND DISCUSSION
1. Effect of Catalytic System Composition and Reaction Medium Composition on the Catalyst Activity and the Molecular-Weight Characteristics of Polyhexene upon Polymerization on Supported TMC
A highly active supported titanium–magnesium catalyst (TMC), which is commonly used for the stereospecific polymerization of propylene, was used as a catalyst [10]. The composition of TMC containing TiCl4 on highly dispersed magnesium dichloride (a support) and dibutyl phthalate (DBP, a stereoregulating electron-donor compound), is usually denoted as TiCl4/MgCl2 ⋅ nDBP (Ti and DBP contents, 2.5 and 12 wt %, respectively). The catalyst is used in polymerization in combination with an organoaluminum cocatalyst, triethylaluminum (TEA) or triisobutylaluminum (TIBA). The cocatalyst is usually introduced into the polymerization medium in combination with an additional electron-donating stereoregulating compound (external donor D); alkylalkoxysilanes RnSi(OR')4 – n are used as these compounds. Thus, the overall composition of the catalytic system used can be represented as follows:
The experimental procedure of the polymerization of 1-hexene on the supported titanium–magnesium catalyst and the determination of the catalyst activity and the molecular-weight characteristics of the polyhexene obtained were described in detail previously [10–13].
This section discusses the effects of the composition of a cocatalyst (TEA or TIBA) and the presence of an external donor (D) and hydrogen, which is commonly used in the polymerization of olefins as a chain-transfer agent for reducing the molecular weight of the resulting polymers, during polymerization on the activity of the catalyst and the molecular-weight characteristics and isotacticity of polyhexene.
1.1. Polymerization of 1-hexene with different cocatalysts (TEA and TIBA). Organoaluminum cocatalysts, in particular, trialkylaluminums, are an essential component of the Ziegler–Natta catalytic systems, including titanium–magnesium catalysts. The active sites of TMC—alkylated surface compounds of trivalent titanium—are formed with their participation. In addition, trialkylaluminums (in particular, AlEt3) are effective polymer chain-transfer agents. In the case of ethylene polymerization on TMC in the absence of hydrogen at a low ethylene pressure, chain transfer with AlEt3 is the predominant chain-transfer reaction, which determines the molecular weight of polyethylene under these conditions [14]. Moreover, it was found previously [14, 15] that the interaction of AlEt3 with TMC active sites during ethylene polymerization facilitated the reversible transformation of active sites into a temporarily inactive state. These processes can lead to changes in the molecular weight and molecular-weight distribution (MWD) of the resulting polymer [14] and to change the number of active sites with varying the polymerization temperature [15]. Trialkylaluminums also participate in side reactions, which occur under the conditions of polymerization on TMC and lead to changes in the composition of surface compounds, the number of active sites, and the activity of the catalysts.
Table 1 summarizes data on the influence of the nature of a cocatalyst on the activity and the molecular-weight characteristics of polyhexene obtained by polymerization on TMC at 70°C. It can be seen that the activity of TMC with a TIBA cocatalyst was significantly higher than the activity on polymerization with a TEA cocatalyst. Even more significant differences were observed upon a comparison between the molecular weights and MWDs of polyhexene obtained in the presence of these cocatalysts. Thus, with the TIBA cocatalyst, a polymer with a very high molecular weight and a narrow MWD was formed (Mw = 2100 kg/mol, Mw/Mn = 3.1). The use of TEA as a cocatalyst led to a sharp decrease in the molecular weight of polyhexene (Mw = 290 kg/mol) and a significant broadening of the MWD (Mw/Mn = 17) (Fig. 1). These data indicate that TEA is an effective polymer chain-transfer agent in contrast to TIBA. However, the presence of TEA was accompanied by an increase in the heterogeneity of the active sites, which was manifested in the formation of a polymer with a wider MWD. To evaluate the heterogeneity of active sites, useful information can be obtained by deconvolution the MWD curves into individual Flory components, which correspond to the formation of a polymer with the polydispersity Mw/Mn = 2 on individual active sites. Table 2 presents the results of this deconvolution. In the case of the TIBA cocatalyst, these data indicate the presence of three types of active sites on the TMC surface. The use of the TEA as cocatalyst led to a sharp decrease in the molecular weight of polyhexene (by a factor of 3–4.5) formed at these sites and the appearance of two additional Flory components with very low molecular weights (6 and 28 kg/mol), which can be considered as the formation of two additional groups of active sites. We believe that the active sites formed in TMC in the presence of TEA exhibit additional heterogeneity in a chain-transfer reaction due to a temporary stop in the growth reaction on some of the active sites upon the reversible adsorption of TEA on them. This process leads to the formation of a low-molecular-weight polymer at these centers, which manifests itself in the broadening of MWD due to the formation of additional low-molecular-weight Flory components. This conclusion is consistent with the results obtained earlier upon ethylene polymerization on supported TMC under conditions when chain transfer in the presence of TEA is the predominant polymer chain-transfer reaction [14].
1.2. Effect of hydrogen on the activity and molecular-weight characteristics of polyhexene. Hydrogen is the most effective chain-transfer agent commonly used in the catalytic polymerization of olefins for regulating the molecular weight of polyolefins. The introduction of hydrogen during the polymerization of ethylene on TMC led to a decrease in the rate of polymerization, whereas an increase in the rate of polymerization was observed in the polymerization of propylene on TMC.
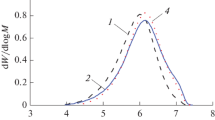
Table 3 summarizes data on the effect of hydrogen on the activity of the catalyst and the molecular weight and MWD of polyhexene obtained on TMC with TEA and TIBA cocatalysts. It is evident that the introduction of hydrogen into the reaction medium in both cases led to a sharp increase in the activity (by a factor of 5–8) and a noticeable decrease in the molecular weight of polyhexene. The polydispersity of polyhexene (Mw/Mn) remained almost unchanged in the case of the TIBA cocatalyst, and it noticeably narrowed in the presence of the TEA cocatalyst. The MWD narrowed due to a predominant decrease in the molecular weight of the high-molecular-weight components, and it was affected to a lesser extent by the low-molecular-weight components (Fig. 2); this fact indicates a higher reactivity of the active sites producing high-molecular-weight polyhexene in the chain-transfer reaction with hydrogen.
MWD curves of polyhexene obtained with the TEA cocatalyst: (1) polymerization in the absence of hydrogen and (2) polymerization in its presence [10].
It was assumed [16, 17] that the increase in activity during propylene polymerization on TMC in the presence of hydrogen is associated with the reactivation of temporarily inactive “sleeping” sites on their interaction with hydrogen. These temporarily inactive sleeping centers are formed after the secondary 2,1-addition of propylene to the growing polymer chain. Interaction with hydrogen at sleeping centers produces titanium hydride compounds, which form active sites containing the Ti–CH2CH2CH3 bond upon the subsequent interaction with the monomer.
A stronger increase in the activity of TMC in the presence of hydrogen (by a factor of 5–8) during the polymerization of 1-hexene, as compared with that in the polymerization of propylene (by a factor of 2–3), indicates a higher probability of the 2,1-addition of 1-hexene to a growing polymer chain and, correspondingly, a higher fraction of sleeping centers in the absence of hydrogen during the polymerization of 1-hexene, as compared with the polymerization of propylene.
1.3. Effect of an external electron-donor compound (D) on the activity of the catalyst and the molecular-weight characteristics and isotacticity of the resulting polyhexene. An external donor (alkylalkoxysilane) is a constituent of the TMC–AlR3 ⋅ mD catalyst system, and it is introduced directly with the cocatalyst into the polymerization reactor. An external donor is responsible for the high stereospecificity of the TiCl4/MgCl2 ⋅ nDBP catalyst and the required high isotacticity of the resulting polymer. Taniike and Terano [18] formulated the ideas on the composition of an external donor and the mechanism of its functioning in propylene polymerization based on the results obtained in a study of the stereospecific polymerization of propylene on TMC.
Echevskaya et al. [12] studied the effect of an external donor during the polymerization of 1-hexene on the activity of TMC and the molecular-weight characteristics and isotacticity of polyhexene (Table 4). With consideration for the above strong effects of the composition of a cocatalyst and hydrogen on the activity and the molecular weight of polyhexene, experiments were carried out with TEA (Table 4, experiment nos. 1–4) and TIBA (Table 4, experiment nos. 5–8) in the absence or in the presence of hydrogen during polymerization in order to study the effect of D. In the case of polymerization with the TEA as cocatalyst in the absence of hydrogen (Table 4, experiment nos. 1 and 2), the introduction of D into the polymerization led to a sharp decrease in the rate of polymerization and a noticeable increase in the molecular weight and isotacticity of the polymer. In the presence of hydrogen (Table 4, experiment nos. 3 and 4), the introduction of D into the polymerization was accompanied by an increase in the activity and a further increase in the isotacticity of polyhexene. It is interesting to note that polyhexene with a maximum isotacticity (95%) was obtained by polymerization in the presence of hydrogen and an external donor (Table 4, experiment no. 4); in the absence of hydrogen, the isotacticity significantly decreased (Table 4, experiment no. 2).
In polymerization with the TIBA cocatalyst in the absence of hydrogen, the introduction of an external donor led to a sharp decrease in the rate of polymerization, an increase in the molecular weight, and a narrowing of the molecular-weight distribution of polyhexene (Table 4, experiment nos. 5 and 6). It can be seen that the narrowing of the MWD of the polymer obtained in experiment no. 6 occurred due to a decrease in the fraction of the low-molecular-weight part of the obtained polymer. It is likely that the rate of polymerization in experiment no. 6 was lower than that in experiment no. 5 due to the predominant deactivation of active sites that produce low-molecular-weight polyhexene; the rate of chain-transfer reaction at the remaining active sites simultaneously decreased.
An external donor is used in the polymerization of propylene on TMC, primarily, to achieve a desired high isotacticity of the polymer. In the case of 1-hexene polymerization, it was found that hydrogen, which was present in the reaction medium as a chain-transfer agent [12] (Tables 4, experiment nos. 4 and 8), made a significant contribution to the high isotacticity of the polymer. It can be seen that a maximum isotacticity of polyhexene (95 or 96.5%) was obtained in experiment nos. 4 and 8 with TEA and TIBA cocatalysts, respectively, which were carried out in the presence of hydrogen and an external donor.
1.4. Effect of polymerization temperature on the molecular-weight characteristics and isotacticity of polyhexene. In the case of the catalytic polymerization of olefins, a decrease in the reaction temperature always leads to an increase in the molecular weight of a polymer. Obviously, this is due to the higher activation energies of polymer chain-transfer reactions compared to the activation energy of a propagation reaction. In accordance with this general statement, Echevskaya et al. [12] found that a decrease in the polymerization temperature from 70 to 30°C in the polymerization of 1-hexene on TMC led to a noticeable increase in the molecular weight of polyhexene. At the same time, changes in the molecular weight and molecular-weight distribution substantially depend on the composition of a catalytic system and the presence of hydrogen during polymerization. (The effect of the reaction temperature on the rate of polymerization with the estimation of effective activation energy will be discussed in Section 2.)
Tables 5 and 6 summarize data on the effect of the polymerization temperature on the molecular weights and MWDs of the polymers obtained upon polymerization with the TIBA (Table 5) and TEA (Table 6) cocatalysts in the absence and presence of an external donor and hydrogen. It can be seen that the nature of a cocatalyst has a decisive influence on the polydispersity of the polymer and the changes in the MWD with a decrease in the polymerization temperature from 70 to 30°C. In the case of polymerization with the TIBA as cocatalyst (Table 5), only a slight tendency to MWD broadening (an increase in Mw/Mn) was observed with a decrease in the polymerization temperature. In this case, the values of Mw and Mn increased by a factor of 2.9–3.8 for various versions of polymerization in the absence or presence of hydrogen and an external donor (series A, B, and C) as the polymerization temperature was decreased from 70 to 30°C. The replacement of the TIBA cocatalyst with TEA led to a sharp change in the polydispersity of polyhexene (Mw/Mn) with the polymerization temperature (Table 6, Fig. 3). In this case, changes in MWD depend on the composition of a catalytic system (the presence of an external donor) and on the presence of hydrogen during the polymerization. In the absence of hydrogen and an external donor (Table 6, series A, experiment nos. 1 and 2), in the presence of hydrogen and an external donor (Table 6, series B, experiment nos. 3 and 4), and in the absence of hydrogen and in the presence of an external donor (Table 6, series C, experiment nos. 5 and 6), a decrease in the polymerization temperature from 70 to 30°C led to a strong broadening of the MWD due to the appearance of a significant fraction of high-molecular-weight polyhexene with the retention of a noticeable fraction of low-molecular-weight polyhexene (Figs. 3a–3c). These data indicate a significant heterogeneity of the active sites of the catalytic system under the experimental conditions of series A–C (Table 6, Fig. 3). The heterogeneity of active sites manifested itself in the fact that the polymerization temperature weakly affected the molecular weight of polyhexene formed on the fraction of active sites producing low-molecular-weight polyhexene and more sharply affected the molecular weight of polyhexene formed on the active sites producing high-molecular-weight polyhexene.
MWD of polyhexene obtained at temperatures of 30 and 70°C in the absence and in the presence of hydrogen and an external donor (TEA cocatalyst [12]): (a) –H2, –D; (b) +H2, +D; (c) –H2, +D; and (d) +H2, –D. Parts (a)–(d) correspond to the series of experiments (A–D) in Table 6.
An exception is the case of polymerization with the TEA cocatalyst in the presence of hydrogen and in the absence of an external donor (Table 6, series D, experiment nos. 7 and 8). A decrease in the polymerization temperature from 70 to 30°C caused a sharp narrowing of the MWD (a decrease in Mw/Mn from 21.2 to 7.5), which occurred due to a sharp decrease in the fraction of low-molecular-weight polymer with a relatively small increase in the molecular weight of high-molecular-weight polymer (Mw).
Thus, the data presented in this section show that the effect of polymerization temperature on the molecular-weight characteristics of the resulting polyhexene depends on the composition of the cocatalyst, and it is also largely determined by the composition of the catalytic system (the presence of an external donor) and, to an even greater extent, by the presence of hydrogen in the reaction medium.
1.5. Possibilities for controlling the activity of TMC in the polymerization of 1-hexene and the molecular-weight characteristics and isotacticity of polyhexene. The results presented in Sections 1.1–1.3 show that the activity of TMCs mainly depends on the composition of the cocatalyst and the presence of hydrogen in the reaction medium. The activity of the catalysts can be controlled over a wide range acceptable for practical use by varying these parameters. In this case, the choice of the catalytic system composition and the polymerization conditions is primarily determined by the necessity of obtaining a polymer with desired molecular-weight characteristics and isotacticity. As shown in Sections 1.1–1.4, these parameters, in turn, can also be widely regulated by varying the composition of a cocatalyst (TEA or TIBA) and the presence of hydrogen and an external donor in polymerization and by changing the polymerization temperature.
As an example, Table 7 and Fig. 4 illustrate the possibility of obtaining polyhexene with low and ultrahigh molecular weights and narrow and wide MWDs with the retention of high catalyst activity. In particular, the preparation of polyhexene with molecular weights (Mw) from 86 to 10 000 kg/mol and polydispersity values (Mw/Mn) from 3.6 to 21 was exemplified.
MWD curves of polyhexene samples obtained at various compositions of the catalytic system and various polymerization conditions. The numbers of curves correspond to the numbers of experiments in Table 7.
Table 4 summarizes data on the preparation of polyhexene with different isotacticity values (from 56 to 96.5%). For greater clarity, Fig. 5 shows the effects of the composition of a cocatalyst and the presence of an external donor and hydrogen in the case of 1-hexene polymerization at various temperatures [12]. Note that polyhexene with high isotacticity (95–97%) can be obtained at different polymerization temperatures (70 and 30°C), and the presence of not only an external donor but also hydrogen during polymerization is an important condition.
Effects of the composition of the cocatalysts ( (◼, ◻) TIBA and ( ,
,  ) TEA) the presence of hydrogen and an external donor (D), and the polymerization temperature of (◼,
) TEA) the presence of hydrogen and an external donor (D), and the polymerization temperature of (◼,  ) 70 and (◻,
) 70 and (◻,  ) 30°C on the isotacticity of polyhexene (the pentad content mmmm) [12].
) 30°C on the isotacticity of polyhexene (the pentad content mmmm) [12].
2. Kinetics of 1-Hexene Polymerization on the TiCl4/MgCl2 ⋅ nDBP–AlR3/D Catalytic System
This section discusses the effect of the reaction temperature on the rate of polymerization with the estimation of the effective activation energy of polymerization reaction [11] and the kinetics of polymer chain transfer reactions, which are responsible for the molecular weight of the resulting polymer [13].
2.1. Effect of reaction temperature on the rate of polymerization. Temperature is one of the main parameters that determine the activity of a catalyst and the molecular-weight characteristics of the resulting polymer. Section 1.3 presented data on a significant effect of the polymerization temperature on the molecular weight and the molecular-weight distribution of polyhexene obtained on TMC. Here, we consider the results obtained by Echevskaya et al. [11], who studied the effect of reaction temperature on the rate of polymerization (catalyst activity) and calculated the effective activation energies of polymerization from these data. It should be noted that, in the determination of the average rate of polymerization used to calculate the effective activation energy, it is necessary to take into account changes in the rate of polymerization with reaction time (that is, the shape of kinetic curves). As an example, Fig. 6 shows the kinetic curves of 1-hexene polymerization on the TMC–AlEt3/D catalytic system at 70°C obtained by polymerization in the absence and presence of hydrogen. Polymerization in the absence of hydrogen (Fig. 6, curve 1) occurred with low activity and a slow decrease in the rate of polymerization with time from 10 to 60 min. The rate of polymerization in the presence of hydrogen was very high in the initial period (10 min) with the subsequent noticeable decrease (by a factor of 2.5) as the polymerization time increased from 10 to 60 min. It should be noted that these kinetic curves were plotted based on the results of separate experiments carried out at polymerization times of 10, 30, and 60 min. The average rate of polymerization at each point was calculated from the yield of polymer with consideration for monomer conversion and changes in monomer concentration in each particular experiment. Knowing the shape of the kinetic curves (Fig. 6), we subsequently used a short polymerization time (10 min) in order to determine the average rate of polymerization in experiments performed in the presence of hydrogen at various temperatures. A longer polymerization time (30 min) was used in experiments conducted in the absence of hydrogen at various temperatures. In the determination of the temperature dependence of the rate of polymerization on the TMC–AlEt3/D catalytic system, it was also necessary to take into account the effects of the composition of a cocatalyst and the presence of an external donor and hydrogen on the activity of the catalyst (see Section 1). Therefore, experiments were carried out to determine the rate of polymerization at different temperatures under different conditions with respect to the composition of a cocatalyst and the presence of an external donor and hydrogen. Table 8 summarizes the results of experiments of series 1–4 for the TEA and TIBA cocatalysts.
Kinetic curves of 1-hexene polymerization on the TMC–TEA/D catalytic system [10] during polymerization (1) in the absence of hydrogen and (2) in its presence at a temperature of 70°C.
The above data indicate that, in most cases, an unusual effect of the reaction temperature on the rate of polymerization was observed, when the rate of polymerization significantly decreased as the reaction temperature was increased from 30 to 70°C (Table 8, series 1, experiment nos. 1–3 and 10–12; series 2, experiment nos. 4–6; and series 3, experiment nos. 7–9). It should be noted that this effect was manifested in catalytic systems that differed in the nature of a cocatalyst and in the presence of an external donor and hydrogen. Only in a single case of polymerization with the TIBA cocatalyst in the presence of hydrogen and an external donor (Table 8, series 2, experiment nos. 13–15), the rate of polymerization noticeably increased as the reaction temperature was increased from 30 to 70°C, as is usually the case in the polymerization of ethylene and propylene on titanium–magnesium catalysts of various compositions at these temperatures.
The effective activation energies of polymerization (Eeff) given in Table 8 were calculated from the average rates of 1-hexene polymerization (Rp) at various reaction temperatures presented in the Arrhenius coordinates [11]. Negative values of Eeff (from –2.2 to –22.8 kJ/mol) were obtained in all cases with the anomalous dependence of the rate of polymerization on the reaction temperature (a decrease in the value of Rp with the increase of reaction temperature); a normal positive value of Eeff = 19.9 kJ/mol was obtained only for the case of polymerization with the TIBA cocatalyst in the presence of hydrogen and an external donor (Table 8, experiment nos. 13–15). As noted above, in the polymerization of ethylene and propylene on various TMCs, the rate of polymerization always increased as the reaction temperature was increased from 30 to 80°C. With consideration for this fact, we carried out [11] the polymerization of propylene on the same TMC sample as in the case of 1-hexene polymerization with the TEA cocatalyst and under the same conditions (in the presence and absence of hydrogen and an external donor) as in the case of 1-hexene polymerization (Table 8, series 2, experiment nos. 4–6 and series 3, experiment nos. 7–9). Table 9 (series 2 and 3) summarizes data on the polymerization of propylene. It can be seen that, in contrast to 1-hexene polymerization, the rate of propylene polymerization significantly increased with an increase in the reaction temperature from 30 to 70°C, and the values of Eeff calculated from these rates according to the Arrhenius equation were 45.2 and 31.7 kJ/mol, respectively. They are in good agreement with the published values of Eeff for the polymerization of propylene on Ziegler–Natta catalysts and differ sharply from the results obtained for the polymerization of 1-hexene on the same TMC sample with TEA cocatalyst under similar polymerization conditions (Table 8, series 2 and 3). in which the values of Eeff were –4.9 and –22.8 kJ/mol, respectively.
The data of Table 8 show that changes in the rate of 1-hexene polymerization upon varying the reaction temperature depend in a complex way on a combination of factors such as the composition of a cocatalyst and the presence or absence of an external donor and hydrogen. Obviously, these factors affect the catalyst activity at different reaction temperatures to varying degrees; in most cases, this leads to abnormal negative values of effective activation energies and to significant differences in the values of Eeff calculated on varying these factors from –22.8 kJ/mol for series 3 with the TEA cocatalyst to 19.9 kJ/mol for series 2 with the TIBA cocatalyst (Table 8). It is likely that the experimental results are determined by the strong influence of these factors on the number of active sites. Moreover, in most cases (Table 8), the number of active sites obviously decreases significantly with increasing of the polymerization temperature. It should be noted that the composition of a cocatalyst and the presence of hydrogen are the main factors affecting the number of active sites on varying polymerization temperatures and, accordingly, the value of Eeff in the polymerization of 1-hexene. This can be seen when comparing the values of Eeff in the polymerization with the TEA cocatalyst in the presence of hydrogen (Eeff = –11.3 kJ/mol, Table 8, series 1, experiment nos. 1–3) and in the absence of hydrogen (Eeff = –22.8 kJ/mol, Table 8, series 3, experiment nos. 7–9), as well as when comparing the values of Eeff for polymerization with TIBA in the presence of hydrogen (Eeff = 19.9 kJ/mol, Table 8, series 2, experiment nos. 13–15) and in the absence hydrogen (Eeff = –2.2 kJ/mol, Table 8, series 4, experiment nos. 16–18).
The increase in the activity of TMC on the polymerization of propylene in the presence of hydrogen is a well-known phenomenon. It is assumed that it is associated with the formation of temporarily inactive sleeping sites as a result of the 2,1-addition of propylene to the growing polymer chain [16, 17]. The interaction of hydrogen with these sleeping centers leads to their reactivation as a result of the formation of a highly reactive titanium–hydrogen bond and the subsequent 1,2-insertion of propylene at this bond. Obviously, the formation of temporarily inactive sleeping centers and their reactivation in the presence of hydrogen is also possible in the polymerization of 1-hexene on TMC according to the same mechanism as that in the polymerization of propylene. In this case, the probability of the formation of sleeping centers increases with the polymerization temperature.
The data of Table 8 on the polymerization of 1‑hexene and Table 9 on the polymerization of propylene show that the effect of hydrogen on the rate of polymerization of 1-hexene was much stronger than that in the polymerization of propylene. In the case of 1-hexene polymerization at 70°C, the introduction of hydrogen increased the rate of polymerization by a factor of 60 (Table 8, experiment nos. 3 and 9), whereas the rate of propylene polymerization increased by a factor of only 1.9 (Table 9, experiment nos. 3 and 6).
In the case of 1-hexene polymerization at 30°C, the rate of polymerization increased by a factor of 35 upon the introduction of hydrogen (Table 8, experiment nos. 1 and 7), whereas it remained almost unchanged in the polymerization of propylene upon the introduction of hydrogen (Table 9, experiment nos. 1 and 4). These results show that the fraction of active sites in a sleeping state during the polymerization of 1-hexene in the absence of hydrogen was much higher than that in the polymerization of propylene. In this case, the fraction of sleeping sites in the polymerization of 1-hexene depends on the composition of a cocatalyst and the polymerization temperature. As noted above, the introduction of hydrogen on the polymerization of 1-hexene with TEA led to an increase in the rate of polymerization by a factor of 60 or 35 at a reaction temperature of 70 or 30°C, respectively (Table 8, experiment nos. 3 and 9 or 1 and 7, respectively). In the case of polymerization with TIBA, the introduction of hydrogen increased the rate of 1-hexene polymerization by a factor of 5.1 or 1.8 at a reaction temperature of 70 or 30°C, respectively (Table 8, experiment nos. 15 and 18 or 13 and 16, respectively).
The introduction of an external donor also significantly affected the rate of 1-hexene polymerization at various polymerization temperatures, and the nature of this effect also depended on the composition of a cocatalyst. In the case of polymerization with TEA, the introduction of an external donor at 70°C increased the rate of polymerization by a factor of 1.5 (Table 8, experiment no. 3 and 6) and more noticeably (by a factor of 2) at 30°C (Table 8, experiment nos. 1 and 4). In polymerization with TIBA, the introduction of an external donor at 70°C led to a decrease in the rate of polymerization by a factor of 2.5 (Table 8, experiment nos. 12 and 15), whereas it increased the rate of polymerization at 30°C by a factor of 1.8 (Table 8, experiment nos. 10 and 13).
Thus, each of the parameters varied during the polymerization of 1-hexene on TMC (the composition of a cocatalyst, hydrogen, and an external donor) in different combinations and at different temperatures have diverse effects on the number of active sites and the rate of polymerization, and, in particular, on the possibility of increasing the number of active sites when lowering the polymerization temperature from 70 to 30°C. Ultimately, all of these factors lead, firstly, to the appearance of anomalous (negative) effective activation energies of polymerization in most cases and, secondly, to a significant difference between the calculated values of Eeff for different compositions of the catalytic system and the reaction medium. In Table 8, the calculated values of Eeff ranged from –22.8 to 19.9 kJ/mol.
Echevskaya et al. [11] also noted that an additional factor responsible for an increase in the rate of polymerization with a decrease in the reaction temperature from 70 to 30°C can be the more complete fragmentation of a supported catalyst by high-molecular-weight polyhexene formed at lower polymerization temperatures. In this work, we found experimentally that, during the polymerization of 1-hexene on TMC, the more efficient fragmentation of a catalyst particle occurred, as compared to the polymerization of propylene on the same catalyst. Similar data on the fragmentation of TMC particles by high-molecular-weight polyhexene formed at 40°C were obtained by Yang et al. [9].
2.2. Kinetics of polymer chain growth-restriction reactions in the polymerization of 1-hexene on TMC. The molecular weight of polymer in catalytic polymerization is determined by a ratio between the rate of propagation reaction and the sum of the rates of chain transfer reactions. After a chain transfer reaction, the active center retains its reactivity, and an unlimited number of polymer macromolecules can be obtained on one active center in the absence of irreversible deactivation reactions of active sites. Thus, in addition to the rate of polymerization, which is determined by the propagation rate constant of polymer chain and the number of active sites, the molecular weight of polymer is an important kinetic parameter of the polymerization process. Under certain conditions, the rate constants of individual reactions occurring in the course polymerization, namely, the rate constants of polymer chain propagation and chain transfer, can be calculated with the use of this parameter.
Now, we discuss the results obtained previously [13] in a study of polymer chain transfer reactions in the polymerization of 1-hexene on a highly active catalytic system including the supported TMC TiCl4/MgCl2 ⋅ nDBP (DBP is dibutyl phthalate) in combination with a TEA or TIBA cocatalyst and an additional stereoregulating component D (D is propyltrimethoxysilane). In Sections 1 and 2.1, we considered information on the polymerization of 1-hexene on this catalytic system and the possibilities of controlling the activity (rate of polymerization) and the molecular weight, molecular-weight distribution, and isotacticity of polyhexene upon varying the catalytic system composition. These data allow us to formulate a number of the following starting points necessary for a study of polymer chain transfer reactions on the polymerization of 1-hexene with the determination of the rate constants of individual chain-transfer reactions:
(1) In the case of 1-hexene polymerization on TMC, the composition of the cocatalyst (TEA or TIBA) has a significant effect on the molecular-weight distribution of the polymer, in contrast to the polymerization of ethylene and propylene on these catalysts. In particular, polyhexene with a wide MWD is formed with the TEA cocatalyst, and the polydispersity of the polymer (Mw/Mn) noticeably changed upon varying the compositions of a catalytic system and a reaction medium.
(2) In contrast to TIBA, TEA is an effective polymer chain transfer agent and a polymer with a reduced molecular weight is formed upon polymerization in the presence of TEA.
(3) Hydrogen serves as an effective polymer chain transfer agent, and the molecular weight of the polymer formed in the polymerization of 1-hexene on TMC sharply decreases upon its introduction.
(4) Available data suggest that chain transfer reactions with TEA, the monomer, and hydrogen can occur in the polymerization of 1-hexene on TMC at a temperature of 70°C.
For the kinetic analysis of chain transfer reactions and the calculation of a ratio between the rate constants of chain transfer and propagation reactions, the following well-known equations, which determine the degree of polymerization Pn (a ratio of the number-average molecular weight Mn to the molecular weight of the monomer) as a ratio of the rate of chain propagation reaction (Rp) to the sum of the rates of chain transfer reactions (Rt), are used:
In the case of 1-hexene polymerization without hydrogen, expressions (1) and (2) can be represented in the form of Eqs. (3) and (4).
where Kg is the rate constant of propagation (L mol–1 s–1), \(K_{{\text{g}}}^{{{\text{Al}}}}\) is the rate constant of chain transfer with TEA or TIBA trialkylaluminum (L mol–1 s–1), \(K_{{\text{p}}}^{{\text{m}}}\) is the rate constant of chain transfer with 1-hexene monomer (L mol–1 s–1), Cp is the concentration of catalyst active sites (mol/molTi), Cm is the monomer concentration (mol/L), and СAl is the concentration of TEA or TIBA trialkylaluminum (mol/L).
In the case of 1-hexene polymerization in the presence of hydrogen, when an additional chain transfer reaction with hydrogen appears, the degree of polymerization is determined by the expression
where \(K_{{\text{t}}}^{{\text{H}}}\) is the rate constant of chain transfer with hydrogen (L mol–1 s–1), CH is the concentration of hydrogen (mol/L), and \(P_{{\text{n}}}^{0}\) is the degree of polymerization of polyhexene obtained by polymerization in the absence of hydrogen.
We consider previous experimental data [13] on the determination of polymerization conditions under which these reactions occur and the estimation of chain transfer rate constants for these reactions.
2.2.1. Polymerization of 1-hexene on TMC with the TIBA cocatalyst in the absence of hydrogen.Table 10 summarizes data on the effect of 1-hexene concentration on the molecular weight and polydispersity (Mw/Mn) of polyhexene obtained on TMC with the TIBA cocatalyst without hydrogen. It can be seen that the molecular weight of polyhexene markedly increased as the monomer concentration was increased from 0.25 to 0.5 mol/L; however, it changed only slightly with a further increase in the monomer concentration to 2 mol/L. The polymer polydispersity (Mw/Mn) decreased with increasing monomer concentration. An analysis of the MWD curves of the polymers obtained at various monomer concentrations showed that the polydispersity of these polymers narrowed mainly due to a decrease in the low-molecular-weight fraction of polymers [13].
The above results of the influence of monomer concentration on the molecular weight of the resulting polymers indicate the occurrence of two chain transfer reactions under these conditions.
(1) A chain transfer reaction with trialkylaluminum noticeable at a monomer concentration of <0.5 mol/L. In this area, an increase in the molecular weight of the polymer with the monomer concentration is observed (Table 10, experiment nos. 1 and 2).
(2) A chain transfer reaction with a monomer prevailing at a monomer concentration of ≥1 mol/L. In this area, the molecular weight of polyhexene remains almost unchanged with increasing monomer concentration (Table 10, experiment nos. 3 and 4). Thus, over a wide range of monomer concentrations (0.25–2 mol/L), the degree of polymerization is determined by Eq. (4), which takes into account the occurrence of two chain transfer reactions under these conditions. At the same time, the data presented in Table 10 indicate changes in the contributions of each of these transfer reactions to the molecular weight of the polymer upon changes in the monomer concentration. This leads to a complex (nonlinear) shape of the dependence of the degree of polymerization on monomer concentration constructed (Fig. 7) based on the data of Table 10 in accordance with Eq. (4). This shape of this dependence makes it impossible to calculate ratios between the rate constants of chain transfer and chain propagation (\({{K_{{\text{t}}}^{{{\text{Al}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{Al}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) and Km/Kp) according to Eq. (4). In this case, the ratio between these constants can be approximately estimated from data on the molecular weights (degrees of polymerization) of polymers obtained under polymerization conditions when one of these chain transfer reactions predominates. Echevskaya et al. [13] calculated the ratios \({{K_{{\text{t}}}^{{{\text{Al}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{Al}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) and Km/Kp between the constants according to Eqs. (6) and (7) based on the data of Table 10 for experiment nos. 1 (Сm = 0.25 mol/L, the chain transfer reaction with TIBA predominates) and 4 (Cm = 2 mol/L, the chain transfer reaction with the monomer predominates).
According to these calculations, the ratios \({{K_{{\text{t}}}^{{{\text{Al}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{Al}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) and Km/Kp are ≤2 × 10–2 and 1.6 × 10–4, respectively. It can be seen that \(K_{{\text{t}}}^{{{\text{Al}}}}\) is higher than \(K_{{\text{t}}}^{{\text{m}}}\) by approximately two orders of magnitude. However, the concentrations of chain-transfer agents (trialkylaluminum and the monomer) should also be taken into account in the evaluation of a ratio between the rates of chain transfer reactions with trialkylaluminum and the monomer. In particular, for experiment no. 1 in Table 10, the TIBA concentration was 6 × 10–3 mol/L at a monomer concentration of 0.25 mol/L. In this case, the ratio of rate of chain transfer reaction with TIBA (\(R_{{\text{t}}}^{{{\text{Al}}}}\)) to the propagation reaction rate (Rp) was 4.8 × 10–4, and the ratio of the rate of chain transfer reaction with the monomer (\(R_{{\text{t}}}^{{\text{m}}}\)) to propagation the rate reaction was 1.6 × 104. The ratio \({{R_{{\text{t}}}^{{{\text{Al}}}}} \mathord{\left/ {\vphantom {{R_{{\text{t}}}^{{{\text{Al}}}}} {R_{{\text{t}}}^{{\text{m}}}}}} \right. \kern-0em} {R_{{\text{t}}}^{{\text{m}}}}}\) = 3 obtained in this calculation shows that, under the conditions of experiment no. 1 (Table 10), the contribution of a chain transfer reaction with TIBA significantly exceeded the contribution of a chain transfer reaction with the monomer. Under the conditions of experiment no. 4 (Table 10), the monomer concentration was 2 mol/L. In this case, a similar calculation led to the ratio \({{R_{{\text{t}}}^{{\text{m}}}} \mathord{\left/ {\vphantom {{R_{{\text{t}}}^{{\text{m}}}} {R_{{\text{t}}}^{{{\text{Al}}}}}}} \right. \kern-0em} {R_{{\text{t}}}^{{{\text{Al}}}}}}\) = 2.7, which is consistent with the predominant contribution of a chain transfer reaction with the monomer to the molecular weight of the polymer.
2.2.2. Polymerization of 1-hexene on TMC with the TIBA cocatalyst in the presence of hydrogen.Table 11 illustrates the effect of hydrogen content during the polymerization of 1-hexene on the molecular weight and molecular-weight distribution of polyhexene. As noted in Section 1.3, the introduction of hydrogen led to a sharp increase in the yield of polymer and a noticeable decrease in the molecular weight of the polymer (Table 11, experiment nos. 1 and 2). An increase in the hydrogen content was accompanied by a further decrease in the molecular weight of the polymer, and the polydispersity of the polymer (Mw/Mn) did not noticeably change. The first order of chain transfer reaction with hydrogen was calculated from the molecular weights of polymers (Table 11) [13]; on this basis, the ratio of the rate constant of chain transfer with hydrogen (\(K_{{\text{t}}}^{{\text{H}}}\)) to the rate constant of growth (Kp) was calculated using Eq. (5) (\({{K_{{\text{t}}}^{{\text{H}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{\text{H}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 0.23). This value is approximately an order of magnitude higher than \({{K_{{\text{t}}}^{{{\text{Al}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{Al}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 2 × 10–3 for the transfer reaction with TIBA and three orders of magnitude higher than \({{K_{{\text{t}}}^{{\text{m}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{\text{m}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 1.6 × 10–4 for the chain transfer reaction with the monomer.
2.2.3. Polymerization of 1-hexene with the TEA cocatalyst in the absence of hydrogen. As was noted in Section 1.1, the replacement of the TIBA cocatalyst by TEA in the polymerization of 1-hexene led to a sharp decrease in the molecular weight of polyhexene. It is obvious that, during the polymerization of 1-hexene on TMC, TEA is a significantly more efficient chain-transfer agent than TIBA, and a change in the concentration of TEA should lead to a change in the molecular weight of the resulting polyhexene. Indeed, data in Table 12 (experiment nos. 1–3) indicate that an increase in the concentration of TEA from 3 to 12 mmol/L led to a decrease in the molecular weight of polyhexene (Mn) by a factor of 2.2.
If the chain transfer reaction with TEA under these conditions is a predominant propagation reaction, the degree of polymerization of polymer (Pn) can be determined using the following simple expression:
where n is the order of the chain transfer reaction with respect to TEA. In accordance with Eq. (8), the degree of polymerization under these conditions also depends on the monomer concentration (Cm). Indeed, a decrease in the concentration of 1-hexene from 2 to 1 mol/L (Table 12, experiment nos. 2 and 4) at the same TEA concentration led to a decrease in the value of Mn by a factor of 1.8. These results confirm that the chain transfer reaction with TEA under these experimental conditions (Table 12) is a predominant transfer reaction; in this case, Eq. (8) can be used to calculate the ratio \({{K_{{\text{t}}}^{{{\text{TEA}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{TEA}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}.\) To carry out such a calculation, previously [13], we first determined the order of chain transfer reaction with TEA (the value of n in Eq. (8)) based on the results of the influence of the concentration of TEA on the molecular weight of polyhexene, which were presented in the logarithmic form of Eq. (8). We found that the order of transfer reaction with respect to TEA (n) was 0.5, which corresponds to well-known data on the polymerization of ethylene and propylene on Ziegler–Natta catalysts. With consideration for this fact, we calculated the ratio \({{K_{{\text{t}}}^{{{\text{TEA}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{TEA}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 0.07 according to Eq. (8) for the conditions of experiment no. 3 in Table 12, which was carried out at a maximum concentration of TEA.
2.2.4. Polymerization of 1-hexene on TMC with the TEA cocatalyst in the presence of hydrogen.Table 13 and Fig. 8 illustrate the effect of hydrogen content on the yield of polymer and the molecular weight and MWD of polyhexene obtained on TMC with the TEA cocatalyst. As shown in Section 1.2, the introduction of hydrogen sharply increased the yield of the polymer, decreased the molecular weight of the polymer, and narrowed the MWD of polyhexene (Table 13, experiment nos. 1 and 2). An increase in the hydrogen content led to a further decrease in the molecular weight of the polymer and a narrowing of the MWD (Table 13, Fig. 8). Moreover, as the hydrogen content was increased from 0.1 to 1.0 bar, the molecular weight of polyhexene decreased due to a decrease in the value of Mw with a slight change in the value of Mn (Table 13, experiment nos. 2–5). These data and the shape of the MWD curves (Fig. 8) indicate that the observed narrowing of the MWD (a decrease in Mw/Mn) occurred mainly due to a decrease in the fraction of the high-molecular-weight polyhexene. This can be due to the fact that hydrogen effectively interacts with active sites producing high-molecular-weight polyhexene and weakly interacts with active sites producing low-molecular-weight polyhexene. Note that such centers producing a low-molecular-weight polymer are absent in the TMC–TIBA catalytic system, on which polyhexene with a narrower MWD (Mw/Mn = 3.6–5.5) is formed both in the absence and in the presence of hydrogen (see Section 1.2). The significant heterogeneity of active sites in the TMC–TEA catalytic system in the chain transfer reaction with hydrogen makes it impossible to correctly determine the value of \({{K_{{\text{t}}}^{{\text{H}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{\text{H}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) for this catalytic system by processing experimental data on the effect of hydrogen content on the value of Mn (Table 13) using Eq. (5).
2.2.5. Conclusions to Section 2.2.Table 14 summarizes the ratios of the rate constants of various polymer chain transfer reactions to the propagation rate constant in the polymerization of 1-hexene on TMC with TIBA and TEA cocatalysts. As can be seen, the rate constants of the chain transfer reactions in the polymerization of 1-hexene increase in the order \(K_{{\text{t}}}^{{\text{m}}}\)\( \ll \)\(K_{{\text{t}}}^{{{\text{TIBA}}}}\) < \(K_{{\text{t}}}^{{{\text{TEA}}}}\) < \(K_{{\text{t}}}^{{\text{H}}}.\) In this case, the sharpest increase occurred on going from \(K_{{\text{t}}}^{{\text{m}}}\) to \(K_{{\text{t}}}^{{{\text{TIBA}}}}\) (by approximately two orders of magnitude) and on going from the quantities \(K_{{\text{t}}}^{{{\text{TIBA}}}}\) and \(K_{{\text{t}}}^{{{\text{TEA}}}}\) to \(K_{{\text{t}}}^{{\text{H}}}.\)
However, it should be noted that the molecular weight of the resulting polymer is ultimately determined not only by the rate constants of propagation and chain transfer reactions but also by the concentration of the compounds involved in these reactions. In particular, as shown in Section 2.2.1, on comparing the rates of chain transfer reactions with the monomer and TIBA, the rate of chain transfer reaction with the monomer is higher at a high monomer concentration (2 mol/L), as compared to the rate of chain transfer reaction with TIBA, due to the very low concentration of TIBA (6 mmol/L), as compared with the monomer concentration (2 mol/L).
The high \({{K_{{\text{t}}}^{{\text{H}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{\text{H}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) ratio leads to a sharp decrease by a factor of 5–6 in the molecular weight of polyhexene upon polymerization with the TIBA cocatalyst (Table 11, experiment nos. 1 and 5) even at a relatively low concentration of hydrogen (5.5 × 10–3 mol/L) in a reactor.
Polymerization with the TEA cocatalyst is a more complicated case from the point of view of regulating the molecular-weight characteristics of polyhexene. Triethylaluminum is an effective polymer chain-transfer agent (high value of \({{K_{{\text{t}}}^{{{\text{TEA}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{TEA}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\)). Moreover, in the presence of TEA, polyhexene with a broader MWD was formed due to the appearance of an additional low-molecular-weight component in the overall MWD. In addition, in this case, the polydispersity of the polymer decreased markedly on polymerization in the presence of hydrogen due to the selective interaction of hydrogen with a part of the active sites producing high-molecular-weight polyhexene.
It is interesting to compare the ratios of the rate constants of polymer chain transfer (Kit) to the propagation rate constant (Kp) obtained in the polymerization of 1-hexene on TMC (Table 14) with the ratios \({{K_{{\text{t}}}^{i}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{i}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) for the polymerization of ethylene on TMC. According to published data on ethylene polymerization [14], these values are \({{K_{{\text{t}}}^{{{\text{TEA}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{\text{TEA}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 1.6 × 10–3, \({{K_{{\text{t}}}^{{{{{\text{C}}}_{2}}{{{\text{H}}}_{4}}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{{{{\text{C}}}_{2}}{{{\text{H}}}_{4}}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 1.2 × 10–5, and \({{K_{{\text{t}}}^{{\text{H}}}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{{\text{H}}}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) = 1.5 × 10–2. It can be seen that, in the case of ethylene polymerization, all of the values of \({{K_{{\text{t}}}^{i}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{i}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) were significantly lower (by a factor of 13–40), as compared with the corresponding values of \({{K_{{\text{t}}}^{i}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{i}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) for the case of 1-hexene polymerization (Table 14). Obviously, the main reason for this is a higher value of the propagation rate constant Kp in the polymerization of ethylene, as compared with an analogous value in the polymerization of 1-hexene.
At the same time, as noted in Section 2.2.1, the concentrations of compounds involved in chain propagation and chain transfer reactions should also be taken into account to evaluate a ratio between the rates of chain transfer reactions (\(R_{{\text{t}}}^{i}\)) and chain propagation reaction (Rp), which ultimately determines the molecular weight of the polymers obtained. In this regard, it is necessary to pay attention to different phase states of the reaction medium during the formation of these polymers. A semicrystalline polymer powder was obtained upon the suspension polymerization of ethylene and propylene in a hydrocarbon diluent (heptane) at polymerization temperatures of no higher than 90°C. In this case, a semicrystalline polymer layer insoluble in heptane was formed on the surface of a catalyst particle in the course of polymerization, and the rates of reactions on the catalyst surface depends on the concentrations of reactants in the near-surface layer of the polymer, which can be significantly lower than the concentrations of these reactants in the reaction medium (heptane). In the polymerization of 1‑hexene on TMC at the same polymerization temperatures, an amorphous polymer, which is soluble in the reaction medium (heptane), was formed. In this case, the concentrations of the reactants at the surface of the catalyst can be close to the concentrations of these reactants in a heptane medium; therefore, these concentrations can be significantly higher than those on the polymerization of ethylene or propylene. However, in all cases (the polymerization of 1‑hexene, ethylene, or propylene), the concentrations of reactants in the reaction medium (heptane) are used to calculate the rates and rate constants of polymer growth and chain transfer reactions. Thus, it can be assumed that the values of \({{K_{{\text{t}}}^{i}} \mathord{\left/ {\vphantom {{K_{{\text{t}}}^{i}} {{{K}_{{\text{p}}}}}}} \right. \kern-0em} {{{K}_{{\text{p}}}}}}\) calculated for the polymerization of 1-hexene are the most correct kinetic characteristics, as compared with those calculated for the polymerization of ethylene and propylene.
CONCLUSIONS
The results of publications [10–13] on the polymerization of 1-hexene on a complex titanium–magnesium catalytic system, which is commonly used for the stereospecific polymerization of propylene, discussed in this review are indicative of significant differences in the kinetics of polymerization of 1-hexene, as compared with the polymerization of propylene and ethylene and, correspondingly, differences in methods used for controlling the rate of polymerization and the molecular weight and molecular-weight distribution of polyhexene.
(1) The most noticeable difference is manifested in a stronger effect of the composition of a cocatalyst (trialkylaluminums) on the rate of polymerization and the molecular weight and molecular-weight distribution of the polymer. In the case of polymerization of 1‑hexene with the TIBA cocatalyst, the activity of the catalyst is usually higher, as compared with TEA. The composition of the cocatalyst even more sharply affected the molecular weight and MWD of polyhexene. In the polymerization of 1-hexene, TEA is an effective chain transfer agent, and a polymer with a significantly lower molecular weight and wider MWD is formed in its presence, as compared with the polyhexene obtained with the TIBA cocatalyst. It is assumed that different phase compositions of the reaction medium are a possible cause of differences in the results obtained upon the polymerization of 1-hexene from the well-known data on the polymerization of ethylene and propylene on TMC. On the polymerization of 1-hexene, an amorphous polymer, which is soluble in the reaction medium (heptane), is formed, whereas semicrystalline insoluble polymer particles with catalyst particles in inside are formed on the polymerization of ethylene and propylene under the same conditions. Obviously, the concentration of compounds (a monomer, trialkylaluminums, etc.) involved in the polymerization on the surface of the catalyst particles can be significantly higher in a “homogeneous” medium (polyhexene solution), as compared to a “heterogeneous” medium (a semicrystalline polymer layer covering catalyst particles). In particular, in the polymerization of 1-hexene, an increased surface concentration of TEA leads to a higher rate of chain transfer reaction with TEA and a noticeable decrease in the molecular weight of the polymer. For the same reason, the probability of the formation of temporarily inactive sites increases due to the reversible adsorption of TEA on active sites, which leads to an MWD broadening due to an increase in the fraction of low-molecular-weight polymer formed at these sites.
(2) Another specific feature of the polymerization of 1-hexene on TMC is an exceptionally strong effect of hydrogen on the activity of the catalyst. When hydrogen is introduced into the reaction medium, the activity of TMC increases by a factor of 5–8 in the polymerization of 1-hexene, whereas the activity increases by a factor of only 2–3 in the polymerization of propylene on the same catalyst. A stronger increase in the activity of the catalyst in the presence of hydrogen indicates a higher probability of the 2,1-addition of 1-hexene to the growing polymer chain and, correspondingly, a higher fraction of “dormant” centers in the absence of hydrogen during the polymerization of 1-hexene, as compared to the polymerization of propylene.
A noticeable effect of hydrogen on the isotacticity of polyhexene was also found. Thus, it was shown that, under various polymerization conditions (TEA and TIBA cocatalysts and polymerization temperatures of 30 and 70°C), a high polymer isotacticity (95–97%) is achieved by polymerization of 1-hexene on TMC due to the presence of not only an external donor but also hydrogen.
(3) The activity of the catalyst and the molecular-weight characteristics of polyhexene also depend on the presence of an external donor (alkylalkoxysilane), which is used in the polymerization of α-olefins on TMC to achieve high isotacticity of the polymer, in the catalytic system. In the polymerization of 1-hexene, the presence of an external donor often leads to a decrease in the rate of polymerization, an increase in the molecular weight of the polymer, and a narrowing of the MWD of the polymer. Moreover, the quantitative values of this effect depend on the cocatalyst (TEA or TIBA), the presence of hydrogen, and the polymerization temperature.
(4) The significant effects of the catalytic system composition and hydrogen on the rate of polymerization and the molecular weight and MWD of polyhexene indicate the need for kinetic studies of various compositions of the catalytic system and the reaction medium. This is also confirmed by experimental data on the effect of the reaction temperature on the rate of 1-hexene polymerization. It was found that the effective activation energy of polymerization (Eeff), calculated from the Arrhenius dependences of the rates of polymerization in a range of 30–70°C, depends on the composition of the catalytic system (the nature of the cocatalyst and the presence of an external donor) and the presence of hydrogen during polymerization and varies widely from a negative (–23 kJ/mol) to a positive value (20 kJ/mol). These values differ significantly from the activation energies of propylene polymerization on the same catalyst (32–45 kJ/mol). The results of an increase in the rate of 1-hexene polymerization with decreasing polymerization temperature from 70 to 30°C are particularly unusual. Obviously, factors such as the composition of the cocatalyst and its concentration on the catalyst surface during the polymerization of 1-hexene and the presence of an external donor and hydrogen affect not only the number of active sites of the catalyst and, accordingly, the rate of polymerization but also changes in the number of active sites with the polymerization temperature. From the data obtained, it follows that the most noticeable decrease in the number of active sites and, accordingly, the rate of polymerization occurs when the reaction temperature is increased from 30 to 70°C in the case of polymerization with the TEA cocatalyst in the absence of hydrogen and in the presence of an external donor (Eeff = –23 kJ/mol). On the contrary, in the case of polymerization with the TIBA cocatalyst in the presence of hydrogen and in the absence of an external donor, the rate of polymerization increases with increasing reaction temperature from 30 to 70°C and the quantity Eeff has a “normal” positive value of 20 kJ/mol.
(5) A detailed kinetic analysis of the polymer chain transfer reactions during the polymerization of 1-hexene was performed. Based on the experimental data on the role of the concentrations of monomer, TEA, and hydrogen during polymerization with TEA and TIBA cocatalysts, the polymerization conditions under which possible chain transfer reactions affect the molecular weight of polyhexene were determined:
(i) chain transfer with the monomer (\(K_{{\text{t}}}^{{\text{m}}}\)),
(ii) chain transfer with the TEA cocatalyst (\(K_{{\text{t}}}^{{{\text{TEA}}}}\)),
(iii) chain transfer with the TIBA cocatalyst (\(K_{{\text{t}}}^{{{\text{TIBA}}}}\)),
(iv) chain transfer with hydrogen (\(K_{{\text{t}}}^{{\text{H}}}\)).
With the use of these data, we determined the ratios of the rate constants of individual chain transfer reactions to propagation the rate constant (Kp), which are responsible for the molecular weight of the polymer obtained. The rate constants of chain transfer reactions increase in the order \(K_{{\text{t}}}^{{\text{m}}}\) < \(K_{{\text{t}}}^{{{\text{TIBA}}}}\) < \(K_{{\text{t}}}^{{{\text{TEA}}}}\) < \(K_{{\text{t}}}^{{\text{H}}}.\) It was shown that, in the evaluation of contributions from individual chain transfer reactions to the molecular weight of the polymer, it is necessary to take into account the concentrations of chain transfer compounds involved in these reactions. Only in this case, the contribution of each of these transfer reactions to the molecular weight of the polymer can be realistically evaluated under specific polymerization conditions.
(6) In general, the results presented in the review indicate considerable possibilities for the target-oriented regulation of the activity of the titanium–magnesium catalytic system and the molecular weight, molecular-weight distribution, and isotacticity of the resulting polyhexene. In particular, the possibility of obtaining a high yield of polyhexene at temperatures from 30 to 70°C (up to 200 kgPH gTi–1 h–1 at a monomer concentration of 2 mol/L) and controlling the molecular weight of the polymer from 7 × 104 to ~107 g/mol with different molecular-weight distributions (Mw/Mn = 3.7–33) and isotacticity of 56–97% was demonstrated.
REFERENCES
Chien, J.C.W. and Gong, B.M., J. Polym. Sci. Polym. Chem., 1993, vol. 31, no. 7, p. 1747.
Saxena, P.K., Eur. Polym. J., 1999, vol. 35, no. 7, p. 1313.
Vasilenko, I.V. and Kostjuk, S.V., Polym. Bull., 2006, vol. 57, no. 2, p. 129.
Zhang, L.T., Fan, Z.Q., and Fu, Z.S., Chin. J. Polym. Sci., 2008, vol. 26, no. 5, p. 605.
Fan, Z., Zhang, L., Xia, S., and Fu, Z., J. Mol. Catal. A: Chem., 2011, vol. 351, p. 93.
Rishina, L.A., Lalayan, S.S., Galashina, N.M., Perepelitsina, E.O., Medintseva, T.I., and Kissin, Y.V., Polym. Sci. B, 2014. 56, p. 25.
Ahmadjo, S., Polym. Adv. Technol., 2016, vol. 27, p. 1523.
Ivchenko, P.V., Nifant’ev, I.E., and Tavtorkin, A.V., Pet. Chem., 2016, vol. 56, issue 9, p. 775.
Yang, P.J., Fu, Z.S., and Fan, Z.Q., Mol. Catal., 2018, vol. 447, p. 13.
Echevskaya, L., Matsko, M., Nikolaeva, M., Sergeev, S., and Zakharov, V., Macromol. React. Eng., 2014, vol. 8, p. 666.
Echevskaya, L., Matsko, M., Nikolaeva, M., and Zakharov, V., Macromol. React. Eng., 2018, vol. 12, no. 1, p. 1.
Echevskaya, L., Matsko, M., Nikolaeva, M., and Zakharov, V., Macromol. React. Eng., 2018, vol. 12, no. 3, p. 1.
Echevskaya, L.G., Matsko, M.A., and Zakharov, V.A., Catalysis in Industry, 2019, vol. 11, issue 2, p. 224.
Nikolaeva, M.I., Mikenas, T.B., Matsko, M.A., Echevskaya, L.G., and Zakharov, V.A., J. Appl. Polym. Sci., 2011, vol. 122, p. 3092.
Barabanov, A.A., Sukulova, V.V., Matsko, M.A., and Zakharov, V.A., J. Mol. Catal. A: Chem., 2015, vol. 396, p. 328.
Chadwick, I.C., Miedema, A., and Sudmeijer, O., Macromol. Chem. Phys., 1994, vol. 195, p. 167.
Bukatov, G.D. and Zakharov, V.A., Macromol. Chem. Phys., 2001, vol. 202, p. 2003.
Taniike, T. and Terano, M., Adv. Polym. Sci., 2013, vol. 257, p. 81.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Makhlyarchuk
Abbreviations: PH, polyhexene; TMC, titanium–magnesium catalyst; DBP, dibutyl phthalate; TEA, triethylaluminium; TIBA, triisobutylaluminum; D, an external donor (alkylalkoxysilane); MWD, molecular-weight distribution.
Rights and permissions
About this article
Cite this article
Matsko, M.A., Echevskaya, L.G. & Zakharov, V.A. 1-Hexene Polymerization on a Highly Active Titanium–Magnesium Catalyst. Kinet Catal 61, 40–57 (2020). https://doi.org/10.1134/S002315842001005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315842001005X