Abstract
The following series of copper(II) complexes based on di(o-tolyl)phosphinic acid (HL) and 1,10-phenanthroline (phen), 5-chloro-1,10-phenanthroline (Cl-phen), 4,7-dimethyl-1,10-phenanthroline (dmphen), 2,2′-bipyridine (bipy) is prepared and characterized: [Cu(OH)L]n (1), [Cu(phen)(H2O)L2] (2), [Cu(dmphen)(H2O)L2] (3), [Cu(Cl-phen)(H2O)L2] (4), [Cu(bipy)(H2O)L2] (5). According to the XRD data, the phosphinate anion L– exhibits a monodentate coordination in mononuclear complexes 2–5 and a bidentate-bridge coordination in the polymer complex 1. The cytotoxic activity of the ligands and complexes is studied using the A549 (carcinoma) and MRC5 (non-neoplastic fibroblasts) human lung cell lines. It is shown that the complexes exhibit pronounced dose-dependent cytotoxicity to these cell lines in the 1-50 µM concentration range.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The past decades have witnessed an increased interest in transition metal compounds as potential therapeutic agents [1-4]. Metal-containing compounds are part of effective medicinal and diagnostic products and are highly important for the pharmaceutical industry. For example, cisplatin, carboplatin, and other platinum based coordination compounds are used in the treatment of tumor diseases, auranofin containing gold(I) ions is employed in the case of rheumatoid arthritis, sodium pertechnetate is utilized as radiopharmaceuticals, etc.
Widely discussed cytotoxic properties of coordination compounds [5-7] allow them to be utilized as a starting platform for the development of antitumor drugs. One such successful examples is copper(II) complexes based on 2,2′-bipyridine, 1,10-phenanthroline, and amino acids/acetylacetonates derivatives patented under the name of “Casiopeinas®” [8, 9]. One of such complexes is currently subject of the phase I of clinical trials in Mexico [10].
The obtained results confirmed that copper(II) complexes based on 2,2′-bipyridine and 1,10-phenanthroline are promising objects for the search for new antitumor drugs. When designing such compounds, the following O-donor systems are often used as additional ligands: carboxylic acid derivatives [11-13] (e.g., salicylic acid [14, 15]), N-oxides [16], biologically active ligands (norfloxacin, levofloxacin, indomethacin, doxycycline, tetracycline, etc.) [17-20], chromone [21, 22], etc.). However, only some of these complexes have been studied in detail in terms of their selectivity, and still a smaller number have been studied in vivo to confirm their antitumor activity [23].
In the present work, were prepared and characterized copper(II) complexes based on di(o-tolyl)phosphinic acid and 1,10-phenanthroline/2,2′-bipyridine derivatives. It was shown that the 1,10-phenanthroline containing complexes in the concentration range 1-50 µM exhibit a pronounced cytotoxic non-selective effect on the A549 cell line of human lung carcinoma.
EXPERIMENTAL
The complexes were prepared using commercial copper(II) acetate hydrate (analytical-grade), 1,10-phenanthroline monohydrate, 2,2′-bipyridine, 4,7-dimethyl-1,10-phenanthroline, and 5-chloro-1,10-phenanthroline (reagent-grade).
Synthesis of di(o-tolyl)phosphinic acid (HL). 30% H2O2 (2 mL) and di(o-tolyl)chlorophosphine (1 g, 0.004 mol) were added to a solution of NaOH (1 g, 0.025 mol) in distilled water (30 mL). The reaction mixture was stirred for 1 h at 70 °C, cooled down and supplemented with an aqueous 20% HCl solution up to pH 2. The product was extracted by dichloromethane (2×20 mL), the organic layer was dried over Na2SO4, and filtered. The solvent was removed in a vacuum. The resulting white powder was dried in air. Yield: 81% (0.80 g). Elemental analysis (%), calculated for C14H15O2P: C 68.3, H 6.1; found: C 68.7, H 6.0. 1H NMR (δ, ppm): 7.47-7.32 (m, 3H, Ph), 7.27-7.10 (m, 5H, Ph), 2.32 (s, 6H, CH3). NMR 31P{1H} (δ, ppm): 36.27. IR spectrum, ν, cm–1: 457, 478, 494, 538, 554, 579, 669, 698, 718, 760, 810, 941, 993, 1032, 1047, 1086, 1142, 1204, 1248 ν(P=O), 1277 ν(P=O), 1285 (P=O), 1379, 1452, 1474, 1566, 1595, 1641, 2928, 2963, 3015, 3061, 3443, 3499 (OH).
Synthesis of [Cu(OH)L]n (1). A weighted sample of di(o-tolyl)phosphinic acid (0.040 g, 0.16 mmol) was added to a weighted sample of copper(II) acetate (0.017 g, 0.08 mmol) dissolved in a 4:1 mixture of ethyl alcohol and water (5 mL) upon slight heating up to 50 °C. After a lavender-blue precipitate was formed, the solution was evaporated for 20 min at 70 °C. The precipitate was filtered through a glass filter, washed with 1 mL portions of ethyl alcohol, and dried in air. Some small amount of XRD quality [Cu(OH)L]n crystals were formed in the mother liquor. Yield: 80% (0.022 g). Elemental analysis (%), calculated for C14H15CuO3P: C 51.6, H 4.6; found: C 52.1, H 4.9. IR spectrum (ν, cm–1), main bands: 1125, 1140, 1202, 1275 ν(P=O); 1454, 1474, 1568, 1593 Rring, 2420 ν(O–H⋯O=P); 2924, 2962, 3017, 3061, 3086 ν(C–H); 3375, 3620 ν(O–H).
Synthesis of [Cu(phen)(H2O)L2] (2). A weighted sample of 1,10-phenanthroline (0.020 g, 0.10 mmol) was dissolved in 3 mL ethyl alcohol, supplemented with a weighted sample of copper(II) acetate monohydrate (0.020 g, 0.10 mmol), and dissolved upon heating up to 50 °C. The resulting bright blue solution was supplemented under stirring by a weighted sample of di(o-tolyl)phosphinic acid (0.049 g, 0.20 mmol). Some time after the latter was dissolved, a pale blue precipitate was formed. The precipitate was filtered, washed with several 1 mL portions of ethyl alcohol, and dried in air. Yield: 63% (0.049 g). Elemental analysis (%), calculated for C40H38CuN2O5P2: C 63.9, H 5.1, N 3.7; found: C 63.3, H 5.2, N 4.0. IR spectrum (ν, cm–1), main bands: 1138, 1173, 1202, 1273 ν(P=O); 1425, 1454, 1472, 1495, 1520, 1591, 1632 Rring, 2421 ν(O–H⋯O=P); 2926, 2953, 3009, 3061 ν(C–H); 3462 ν(O–H). TGA: 3.1% mass loss at 175 °C. Calculated for 1H2O: 2.4%.
Synthesis of [Cu(dmphen)(H2O)L2] (3). A weighted sample of 4,7-dimethyl-1,10-phenanthroline (0.010 g, 0.050 mmol) was dissolved in 3 mL ethyl alcohol, supplemented with a weighted sample of copper(II) acetate monohydrate (0.010 g, 0.050 mmol) that was dissolved upon slight heating up to 50 °C. A weighted sample of di(o-tolyl)phosphinic acid (0.025 g, 0.10 mmol) was added to the resulting solution. Some time after it dissolved, a pale green precipitate was formed. The solution with the precipitate was evaporated to half the original volume at 80 °C. The precipitate was filtered, washed by 1 mL portions of ethyl alcohol, and dried in air. Dimethyl sulfoxide (0.50 mL) was added to the mother liquor, and [Cu(dmphen)(H2O)L2]·3H2O (3a) crystals suitable for XRD were formed after a few months, which could not be though collected because of small quantities. Yield: 60% (0.024 g). Elemental analysis (%): calculated for C42H42CuN2O5P2 - C 64.6, H 5.4, N 3.6; found - C 63.7, H 5.8, N 3.9. IR spectrum (ν, cm–1), main bands: 1138, 1163, 1202, 1231, 1273 ν(P=O); 1425, 1454, 1474, 1495, 1526, 1581, 1593, 1610, 1625 Rring, 2413 ν(O–H⋯O=P); 2922, 2960, 3007, 3047 ν(C–H); 3443 ν(O–H). TGA: mass loss 3.2% at 130 °C. Calculated for 1H2O: 2.3%.
Synthesis of [Cu(Cl-phen)(H2O)L2] (4). A weighted sample of 5-chloro-1,10-phenanthroline (0.021 g, 0.10 mmol) was dissolved in ethyl alcohol (3 mL). A weighted sample of copper(II) acetate monohydrate (0.021 g, 0.10 mmol) was added to the obtained solution an dissolved upon slight heating up to 50 °C. A weighted sample of di(o-tolyl)phosphinic acid (0.049 g, 0.20 mmol) was added to the resulting solution. Some time after it dissolved, a pale green precipitate was formed; the solution with the precipitate was evaporated to half the original volume at 80 °C. The precipitate was filtered, washed with 1 mL portions of ethyl alcohol, and dried in air. Yield: 84% (0.066 g). Elemental analysis (%),calculated for C40H37ClCuN2O5P2: C 61.1, H 4.7, N 3.6; found: C 60.6, H 4.9, N 3.8. IR spectrum (ν, cm–1), main bands: 1136, 1171, 1202, 1273 ν(P=O); 1423, 1454, 1520, 1585, 1618 Rring, 2446 ν(O–H⋯O=P); 2922, 2945, 3003, 3057, 3086 ν(C–H); 3470 ν(O–H). TGA: 2.7% mass loss at 180 °C. Calculated for 1H2O: 2.3%.
Synthesis of [Cu(bipy)(H2O)L2] (5). A weighted sample of 2,2′-bipyridine (0.016 g, 0.10 mmol) was dissolved in ethyl alcohol (3 mL). A weighted sample of copper(II) acetate monohydrate (0.021 g, 0.10 mmol) was added to the solution and dissolved upon slight heating up to 50 °C. A weighted sample of di(o-tolyl)phosphinic acid (0.050 g, 0.20 mmol) was added to the obtained solution. After its dissolution, a pale blue precipitate was formed. The solution was filtered through a glass filter, washed with 1 mL portions of ethyl alcohol, and dried in air. After several months, a small amount of [Cu(bipy)(H2O)L2] crystals suitable for XRD were formed in the mother liquor. Yield: 51% (0.039 g). Elemental analysis (%),calculated for C38H38CuN2O5P2: C 62.7, H 5.3, N 3.9; found: C 62.7, H 5.2, N 4.0. IR spectrum (ν, cm–1), main bands: 1138, 1163, 1202, 1253, 1273 ν(P=O); 1447, 1474, 1497, 1568, 1593, 1603, 1608 Rring, 2428 ν(O–H⋯O=P); 2925, 2952, 3011, 3053, 3075 ν(C–H); 3472 ν(O–H). TGA: 4.5% mass loss at 160 °C. Calculated for 1H2O: 2.5%.
The CHN analysis was performed at the Analytical Laboratory of NIIC SB RAS on a Vario MICRO cube analyzer according to the standard procedure. The IR spectra were registered on a Scimitar FTS 2000 FTIR spectrometer in the region of 4000-400 cm–1. The samples were prepared in the form of suspensions in vaseline and fluorinated oil and PE and pressed in KBr pellets. Powder XRD was performed on a Shimadzu XRD-7000S diffractometer (CuKα radiation, λ = 1.54056 Å, Ni-filter, 2θ range from 5° to 40°, counting time per point = 1 s). The studied samples were ground in an agate mortar in the presence of heptane; the resulting suspension was deposited to the polished side of a standard quartz cuvette; after the heptane dried, the sample was a thin (~100 µm) even layer. The thermogravimetric analysis was performed on a NETZSCH TG 209 F1 Iris Thermo Microbalance instrument in helium in open Al2O3 crucibles at a heating rate of 10 °C/min.
XRD. The XRD study of single crystals of 1, 3a, 5 was conducted according to the standard procedure on a Bruker D8 Venture diffractometer (MoKα monochromatic graphite radiation, λ = 0.71073 Å) at 150 K. The measurements were conducted using φ- and ω-scanning. Absorption corrections were introduced using the SADABS program [24]. The structures were solved and refined with the SHELXTL program [25, 26] using the Olex2 interface [27]. Atomic displacement parameters for non-hydrogen atoms were refined anisotropically. Positions of hydrogen atoms were calculated geometrically and refined using a riding model. The outer sphere of 3a contains disordered water molecules. The electron density corresponding to three water molecules in the system was removed by using the Solvent Mask function of the Olex2 program. The crystal data and structure refinements are listed in Table 1. The XRD data were deposited with the Cambridge Crystallographic Data Centre (CCDC No. 2352372-2352374) and can be obtained free of charge at http://www.ccdc. cam.ac.uk/data_request/cif.
Cytotoxic activity. The experiments were conducted with a A549 cell line of human lung carcinoma (Biolot, Russia) and MRC5 non-tumor human lung fibroblasts (State Research Center of Virology and Biotechnology VECTOR, Russia). Cell viability was estimated by the double staining method using Hoechst 33342 and propidium iodide (PI) fluorescent dyes according to the standard procedure described earlier in [28]. The cells were seeded into 96-well plates and cultured in DMEM (MRC5) or DMEM/F12 (A549) media in a CO2 incubator at 37 °C. After 24 h, the compounds dissolved in ethanol in the 0.20-50 µM concentration range were added, and the cells were incubated for 48 h. The cells were stained by Hoechst 33342 (Sigma-Aldrich) and propidium iodide (Invitrogen) for 30 min at 37 °C. The records were performed on an IN Cell Analyzer 2200 (GE Healthcare, UK) in the automatic mode using 4 fields per well. The obtained images were analyzed using the In Cell Investigator program to determine live, dead, and apoptotic cells in the entire population. The result is presented as the percentage of cells from three independent experiments ± standard deviation. The experimental dependence of the percentage of living cells on the compound concentration was approximated by a nonlinear function. The LC50 parameter was calculated as the compound concentration causing 50% cell death.
RESULTS AND DISCUSSION
Di(o-tolyl)phosphinic acid (HL) was prepared by the reaction of di(o-tolyl)chlorophosphine, NaOH, and 30% H2O2 upon heating up to 70 °C, and the pH value of 2 was subsequently achieved by using a 20% hydrochloric acid aqueous solution (Fig. 1). Adding an HL weighted sample to copper(II) acetate dissolved in a water–ethanol mixture resulted in a precipitate of the [Cu(OH)L]n (1) polymer complex. In spite of the Cu(OAc)2:HL = 1:2 molar ratio, the complex contains copper ions and acid in the 1:1 ratio and the hydroxide anion. The [Cu(phen)(H2O)L2] (2), [Cu(dmphen)(H2O)L2] (3), [Cu(Cl-phen)(H2O)L2] (4), [Cu(bipy)(H2O)L2] (5) complexes were prepared by the interaction of copper(II) acetate and the corresponding derivative of 1,10-phenanthroline or 2,2′-bipyridine in ethanol and subsequent addition of a weighted sample of di(o-tolyl)phosphinic acid (1:1:2 molar reactant ratio) to the mixture. HL is deprotonated during the synthesis, so that none of the complexes contains acetate ions.
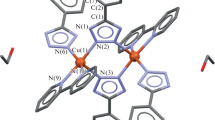
Single crystals of 1, 3a, 5 were prepared by a slow crystallization from water-ethanol mother liquors. According to the XRD data, the L– phosphinate anion in [Cu(OH)L]n (1) is coordinated by oxygen atoms to the copper(II) ion in the bidentate-bridging mode, thus forming polymer chains (Fig. 2). The coordination environment is complemented by the hydroxide ion coordinated in a similar manner. The coordination polyhedron composed of four oxygen atoms is a square, as is confirmed by the parameter τ4 = 0 [29]. The hydroxide ion and an oxygen atom of the L– anion in this compound are connected by an H-bond with distances between oxygen atoms equal to 2.651 Å and 2.660 Å (hereinafter, distances between oxygen atoms are given for H-bonds). The lengths of Cu–O(L–) and Cu–O(OH) bonds are 1.934 Å and 1.897 Å, respectively.
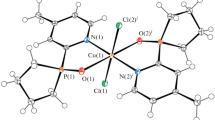
Complexes [Cu(dmphen)(H2O)L2]·3H2O (3a) and [Cu(bipy)(H2O)L2] (5) have a similar structure and are mononuclear; the L– phosphinate anion is monodentate coordinated, while dmphen and bipy chelate the central atom (Fig. 3a). The coordination environment is complemented by a water molecule; the polyhedron is a square pyramid with the τ5 parameter equal to 0.19 (3a) and 0.36 (5) [30]. The complexes contain intramolecular hydrogen bonding between a water molecule and the ligand oxygen with O–H⋯O distances equal to 2.668 Å (3a) and 2.709 (5) Å. Besides, the phenyl group of the L– anion and dmphen/bipy are connected by a π–π interaction with a length of 3.568 Å (3a) and 3.744 Å (5), respectively (Fig. 3a). Two neighboring molecules of the complex (water molecule and oxygen of the phosphinate anion) are connected by intermolecular hydrogen bonds with distances 2.586 Å (3a) and 2.575 Å (5), thus leading to the formation of a non-covalent dimer (Fig. 3b). Such dimers are packed into a polymer chain due to π-staking between dmphen or bipy with a distances 3.532 Å (3a) and 3.694 Å (5) (Fig. 3c). The length of the Cu–O(L–) bond in the complexes is 1.941-2.164 Å, that of Cu–N(dmphen/bipy) is 1.990-2.032 Å, and that of Cu–O(H2O) is 1.981 Å (3a) and 2.031 Å (5).
Structure of [Cu(dmphen)(H2O)L2]·3H2O (3a) with intramolecular hydrogen bonding (green dashed line) and π-staking (blue dashed line) (a); intermolecular H-bond between neighboring molecules (b); packing of non-covalent dimers (different colors) into a polymer chain due to intermolecular π-staking (c) (see the electronic version).
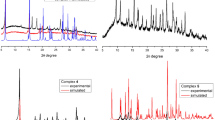
The powder XRD data showed that complexes 1–5 are crystalline, and the XRD patterns of 1, 5 are similar to those calculated from the XRD data, thus confirming their purity (Fig. 4). Complexes 3 and 3a exhibit significant differences in the positions and intensities of reflections, apparently due to the fact that 3a contains three outer-sphere water molecules, significantly affecting the crystal packing of mononuclear complexes.
Compound 1–5 were characterized by IR spectroscopy. The spectra of the complexes have characteristic frequencies assigned to P=O stretchings (strong bands at 1125-1275 cm–1), stretching-deformation vibrations of heterocycles and benzene rings (bands of varying intensity at 1423-1632 cm–1), C–H stretchings of alkyl groups and aromatic rings (weak bands at 2922-3086 cm–1). The complexes contain coordinated water molecules (2–5) or hydroxide-anions (1) manifested in their IR spectra as broad ν(O–H) bands at 3375-3470 cm–1. According to the XRD data, complexes 1–5 contain intra- and intermolecular hydrogen bonds between water molecules and oxygen atoms of di(o-tolyl)phosphinate anions with distances 2.575-2.660 Å. Apparently, these interactions corresponds to the additional wide band of ν(O–H⋯O=P) stretchings at 2413-2446 cm–1 (Fig. 5). Such structure of the high-frequency region of the IR spectrum is typical for phosphinic acids [31-33]. In particular, the spectrum of the dimethylphosphinic acid Me2P(O)OH connected into dimers by strong H-bonds exhibits the ABC structure of the ν(OH) band with maxima at 2800 cm–1 (A), 2400 cm–1 (B), and 1700 cm–1 (C) [31]. Thus, the IR spectroscopy data agree well with the structural data.
Thermal stability of the mixed-ligand complexes 2–5 was studied in the 25-600 °C range. According to the thermogravimetric analysis data, a loss of one coordinated water molecule in the complexes occurs at 160-180 °C (Fig. 6). An exception is compound 3, where this process begins at room temperature and terminates by 130-140 °C. Further decomposition begins at 235 °C for 1,10-phenanthroline based complexes and at 160 °C for the complex 5 with bipy.
The action of di(o-tolyl)phosphinic acid HL and complexes 1–5 on human cell viability was estimated for the following lung cell lines: A549 (carcinoma) and MRC5 (non-tumor fibroblasts). The results are listed in Table 2 for the LC50 parameter calculated as the substance concentration causing 50% cell death.
Exposing the cells to the action of di(o-tolyl)phosphinic acid HL and copper(II) acetate in the studied concentration range (5-100 µM) for 48 h did not change the rate of their proliferation and/or their death (LC50 > 100 µM), while copper complexes 1–5 exhibited both cytostatic and cytotoxic effects (Table 2, Fig. 7). The properties of 2–4, containing 1,10-phenanthroline and its derivatives, are most pronounced: the LC50 values vary from 3.89 µM to 5.2 µM. Compared with the data earlier reported, the cytotoxicity of 1 and 5 is an order of magnitude lower (LC50 ~ 40-50 µM, MRC5 cells).
The cytotoxicity was studied both on human tumor cell lines and on non-tumor ones in order to calculate selectivity indices for the synthesized compounds. The indices were determined as the ratio of LC50 on the MRC5 line to its value on the A549 line. For 2–5, the selectivity indices are close to 0.8-0.9, indicating that these complexes are not selective to the tumor cell line. In the case of 5, fibroblasts are even more sensitive to the complex than tumor cells: the action of 50 µM of the complex for 48 h caused ~75% fibroblast death, whereas the death of A549 increases relative to the control group and reaches ~12% only upon the action of 100 µM of the complex (Fig. 7).
CONCLUSIONS
Copper(II) complexes based on derivatives of 1,10-phenanthroline/2,2′-bipyridine and di(o-tolyl)phosphinic acid were prepared and characterized. It was established that copper has a planar square environment in the [Cu(OH)L]n polymer complex and a distorted square-pyramidal environment in [Cu(LN–N)(H2O)L2] mononuclear complexes. At the same time, the L– phosphinate anion has different coordinations: a bidentate-bridge of coordination in the first case and a monodentate one in other complexes. It was shown that 1,10-phenanthroline based complexes exhibit pronounced dose-dependent cytotoxicity exceeding that of cisplatin on the A549 and MRC5 cell lines, while the cytotoxicity of the 2,2′-bipyridine based complex is an order of magnitude lower. At the same time, none of the compounds of this series demonstrated selectivity to the A549 tumor cell line.
ACKNOWLEDGMENTS
The authors thank A. P. Zubareva and N. N. Komardina for providing the elemental analysis data, V. N. Yudina for the measurements at the XRD Facility of Nikolaev Institute of Inorganic Chemistry, and M. O. Matveeva for conducting the powder XRD analysis.
This work was funded by the Russian Science Foundation (project No. 20-73-10207).
REFERENCES
J. Karges, R. W. Stokes, and S. M. Cohen. Metal complexes for therapeutic applications. Trends Chem., 2021, 3(7), 523-534. https://doi.org/10.1016/j.trechm.2021.03.006
D. Tesauro. Metal complexes in diagnosis and therapy. Int. J. Mol. Sci., 2022, 23(8), 4377. https://doi.org/10.3390/ijms23084377
M. J. S. A. Silva, P. M. P. Gois, and G. Gasser. Unveiling the potential of transition metal complexes for medicine: Translational in situ activation of metal-based drugs from bench to in vivo applications. ChemBioChem, 2021, 22(10), 1740-1742. https://doi.org/10.1002/cbic.202100015
G. Gasser. Metal complexes and medicine: A successful combination. Chimia, 2015, 69(7/8), 442. https://doi.org/10.2533/chimia.2015.442
A. Kumar Singh, A. Kumar, H. Singh, P. Sonawane, P. Pathak, M. Grishina, J. Pal Yadav, A. Verma, and P. Kumar. Metal complexes in cancer treatment: Journey so far. Chem. Biodivers., 2023, 20(4). https://doi.org/10.1002/cbdv.202300061
G. Shumi, T. Desalegn, T. B. Demissie, V. P. Ramachandran, and R. Eswaramoorthy. Metal complexes in target-specific anticancer therapy: Recent trends and challenges. J. Chem., 2022, 2022, 1-19. https://doi.org/10.1155/2022/9261683
U. Ndagi, N. Mhlongo, and M. Soliman. Metal complexes in cancer therapy - an update from drug design perspective. Drug Des. Devel. Ther., 2017, 11, 599-616. https://doi.org/10.2147/dddt.s119488
L. Ruiz-Azuara. Process to Obtain New Mixed Copper Aminoacidate Complexes from Phenanthrolines and Their Alkyl Derivatives to be Used as Anticancerigenic Agents. European Patent 0434445A2, 1991.
L. Ruiz-Azuara. Copper Aminoacidate Diimine Nitrate Compounds and Their Methyl Derivatives and a Process for Preparing Them. US Patent 5576326A, 1996.
Z. Aguilar-Jiménez, A. Espinoza-Guillén, K. Resendiz-Acevedo, I. Fuentes-Noriega, C. Mejía, and L. Ruiz-Azuara. The importance of being casiopeina as polypharmacologycal profile (mixed chelate–copper (II) complexes and their in vitro and in vivo activities). Inorganics, 2023, 11(10), 394. https://doi.org/10.3390/inorganics11100394
C. Slator, Z. Molphy, V. McKee, C. Long, T. Brown, and A. Kellett. Di-copper metallodrugs promote NCI-60 chemotherapy via singlet oxygen and superoxide production with tandem TA/TA and AT/AT oligonucleotide discrimination. Nucleic Acids Res., 2018, 46(6), 2733-2750. https://doi.org/10.1093/nar/gky105
Z. Zhang, H. Wang, Q. Wang, M. Yan, H. Wang, C. Bi, S. Sun, and Y. Fan. Anticancer activity and computational modeling of ternary copper(II) complexes with 3-indolecarboxylic acid and 1,10-phenanthroline. Int. J. Oncol., 2016, 49(2), 691-699. https://doi.org/10.3892/ijo.2016.3542
J. A. Eremina, E. V. Lider, T. S. Sukhikh, L. S. Klyushova, M. L. Perepechaeva, D. G. Sheven′, A. S. Berezin, A. Y. Grishanova, and V. I. Potkin. Water-soluble copper(II) complexes with 4,5-dichloro-isothiazole-3-carboxylic acid and heterocyclic N-donor ligands: Synthesis, crystal structures, cytotoxicity, and DNA binding study. Inorg. Chim. Acta, 2020, 510, 119778. https://doi.org/10.1016/j.ica.2020.119778
M. O′Connor, A. Kellett, M. McCann, G. Rosair, M. McNamara, O. Howe, B. S. Creaven, S. McClean, A. Foltyn-Arfa Kia, D. O′Shea, and M. Devereux. Copper(II) complexes of salicylic acid combining superoxide dismutase mimetic properties with DNA binding and cleaving capabilities display promising chemotherapeutic potential with fast acting in vitro cytotoxicity against cisplatin sensitive and resistant cancer cell lines. J. Med. Chem., 2012, 55(5), 1957-1968. https://doi.org/10.1021/jm201041d
L. Kucková, K. Jomová, A. Švorcová, M. Valko, P. Segľa, J. Moncoľ, and J. Kožíšek. Synthesis, crystal structure, spectroscopic properties and potential biological activities of salicylate–neocuproine ternary copper(II) complexes. Molecules, 2015, 20(2), 2115-2137. https://doi.org/10.3390/molecules20022115
H. S. Moradi, E. Momenzadeh, M. Asar, S. Iranpour, A. R. Bahrami, M. Bazargan, H. Hassanzadeh, M. M. Matin, and M. Mirzaei. Bioactivity studies of two copper complexes based on pyridinedicarboxylic acid N-oxide and 2,2′-bipyridine. J. Mol. Struct., 2022, 1249, 131584. https://doi.org/10.1016/j.molstruc.2021.131584
A. Bykowska, U. K. Komarnicka, M. Jeżowska-Bojczuk, and A. Kyzioł. CuI and CuII complexes with phosphine derivatives of fluoroquinolone antibiotics - A comparative study on the cytotoxic mode of action. J. Inorg. Biochem., 2018, 181, 1-10. https://doi.org/10.1016/j.jinorgbio.2018.01.008
J.N. Boodram, I. J. Mcgregor, P. M. Bruno, P. B. Cressey, M. T. Hemann, and K. Suntharalingam. Breast cancer stem cell potent copper(II)–non-steroidal anti-inflammatory drug complexes. Angew. Chem., Int. Ed., 2016, 55(8), 2845-2850. https://doi.org/10.1002/anie.201510443
P. P. Silva, W. Guerra, J. N. Silveira, A. M. da C. Ferreira, T. Bortolotto, F. L. Fischer, H. Terenzi, A. Neves, and E. C. Pereira-Maia. Two new ternary complexes of copper(II) with tetracycline or doxycycline and 1,10-phenanthroline and their potential as antitumoral: cytotoxicity and DNA cleavage. Inorg. Chem., 2011, 50(14), 6414-6424. https://doi.org/10.1021/ic101791r
M. Bashir and I. Yousuf. Synthesis, structural characterization and in vitro cytotoxic evaluation of mixed Cu(II)/Co(II) levofloxacin–bipyridyl complexes. Inorg. Chim. Acta, 2022, 532, 120757. https://doi.org/10.1016/j.ica.2021.120757
S. Khursheed, M. Rafiq Wani, G. G. H. A. Shadab, S. Tabassum, and F. Arjmand. Synthesis, structure elucidation by multi-spectroscopic techniques and single-crystal X-ray diffraction of promising fluoro/bromo-substituted-chromone(bpy)copper(II) anticancer drug entities. Inorg. Chim. Acta, 2022, 538, 120967. https://doi.org/10.1016/j.ica.2022.120967
J. Vančo, Z. Trávníček, J. Hošek, and Z. Dvořák. Heteroleptic copper(II) complexes of prenylated flavonoid osajin behave as selective and effective antiproliferative and anti-inflammatory agents. J. Inorg. Biochem., 2022, 226, 111639. https://doi.org/10.1016/j.jinorgbio.2021.111639
Y. A. Golubeva and E. V. Lider. Copper(II) complexes based on 2,2′-bipyridine and 1,10-phenanthroline as potential objects for developing antitumor drugs. J. Struct. Chem., 2024, 65(6), 1159-1209. https://doi.org/10.1134/s0022476624060088
APEX2 (Version 2.0), SAINT (Version 8.18c) and SADABS (Version 2.11). Madison, WI, USA: Bruker AXS Inc., 2000-2012.
G. M. Sheldrick. SHELXT - Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053273314026370
G. M. Sheldrick. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71(1), 3-8. https://doi.org/10.1107/s2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr., 2009, 42(2), 339-341. https://doi.org/10.1107/s0021889808042726
J. A. Eremina, E. V. Lider, N. V. Kuratieva, D. G. Samsonenko, L. S. Klyushova, D. G. Sheven′, R.E . Trifonov, and V. A. Ostrovskii. Synthesis and crystal structures of cytotoxic mixed-ligand copper(II) complexes with alkyl tetrazole and polypyridine derivatives. Inorg. Chim. Acta, 2021, 516, 120169. https://doi.org/10.1016/j.ica.2020.120169
L. Yang, D. R. Powell, and R. P. Houser. Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans., 2007, (9), 955-964. https://doi.org/10.1039/b617136b
A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, and G. C. Verschoor. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc., Dalton Trans., 1984, (7), 1349-1356. https://doi.org/10.1039/dt9840001349
K. G. Tokhadze, G. S. Denisov, M. Wierzejewska, and M. Drozd. First example of the ABC ν(OH) absorption structure for both gaseous and crystalline phase: infrared studies of dimethylphosphinic acid. J. Mol. Struct., 1997, 404(1/2), 55-62. https://doi.org/10.1016/s0022-2860(96)09360-x
N. Rekik, H. Ghalla, and G. Hanna. Explaining the structure of the OH stretching band in the IR spectra of strongly hydrogen-bonded dimers of phosphinic acid and their deuterated analogs in the gas phase: A computational study. J. Phys. Chem. A, 2012, 116(18), 4495-4509. https://doi.org/10.1021/jp3016084
R. E. Asfin, G. S. Denisov, and K. G. Tokhadze. The infrared spectra and enthalpies of strongly bound dimers of phosphinic acids in the gas phase. (CH2Cl)2POOH and (C6H5)2POOH. J. Mol. Struct., 2002, 608(2/3), 161-168. https://doi.org/10.1016/s0022-2860(01)00925-5
Funding
This study was funded by the Ministry of Science and Higher Education of the Russian Federation (project No. 121031700321-3). Cytotoxic properties of the considered compounds was studied on the equipment of the Center for Collective Use “Proteomic Analysis” of the Federal Research Center of Fundamental and Translational Medicine and supported by the Ministry of Science and Higher Education of the Russian Federation (project No. 122032200236-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors of this work declare that they have no conflicts of interest.
Additional information
Russian Text © The Author(s), 2024, published in Zhurnal Strukturnoi Khimii, 2024, Vol. 65, No. 7, 132300.https://doi.org/10.26902/JSC_id132300
Publisher’s Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Savinykh, P.E., Golubeva, Y.A., Smirnova, K.S. et al. Synthesis and Structure of Cytotoxic Copper(II) Complexes Based on di(o-tolyl)Phosphinic Acid and 2,2'-Bipyridine/1,10-Phenanthroline. J Struct Chem 65, 1465–1476 (2024). https://doi.org/10.1134/S0022476624070175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476624070175