Abstract
As a result of crystallization from a solution containing Na5[H3PtW6O24] and Cs2SO4, yellow crystals of a complex are obtained. The composition and structure of the complex are determined by single crystal X-ray diffraction (XRD). The complex has the composition Cs8.1Na2.9[(PtW6O24)2H5]·8H2O (1). For the first time, a proton-bound dimer [(PtW6O24)2H5]11–of two Anderson-type anions is found in its structure. Its phase purity is confirmed by powder XRD for a freshly prepared sample. Sample ageing leads to a loss of water molecules and a change in its crystal structure.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Complexes of polyoxometalates (POMs) with noble metals are of great interest due to a unique combination of the reactivity of the polyoxometalate matrix and noble metal. For instance, in these complexes, it is possible to stabilize high oxidation states of noble metal that usually acts as an active intermediate in catalytic oxidation reactions. Moreover, the complexes of this type fit well into the development of the field such as single-atom catalysis [1, 2].
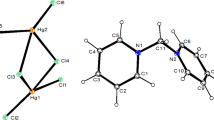
Platinum complexes with POMs are among the most understood [3-5]. The most abundant representative of this family is the Anderson-type anion [PtIVM6O24]8– (M = Mo, W) (Fig. 1). We have recently demonstrated for the first time [6] the possibility of obtaining a trimetallic [Pt{Sb(OH)2}W5O22]8– anion isolated as K6Na2[Pt{Sb(OH)2}W5O22] (NO3)0.1(OH)0.911H2O. During its synthesis the Pt(IV) cation wins the “competition” over Sb(V) for forming an Anderson-type polyanion in its coordination sphere. This fact evidences a significant thermodynamic stability of the [PtW6O24]8– anion. Thanks to the numerous works of Korean Professor U. Lee, two features of the behavior of [PtIVMo6O24]8– anions were discovered: (a) the possibility of existing in the form of two isomers (Fig. 1) and (b) protonation of Pt–O–Mo bridging oxide ligands.
α-[PtIVMo6O24]8– has the point symmetry group D3d, while the β-[PtIVMo6O24]8– symmetry decreases to C2ν [7]. There are significantly less structurally characterized examples of a less symmetrical anion. For instance, the heptamolybdate [Mo7O24]6– anion is a characteristic representative of the family of these structures. In addition, β-[H2SbMo6O24]5– [8] and \(\beta \text{-}[(\text{MoO}_{4}^{2-}){{(\text{O})}_{\text{2}}}(\text{M}{{\text{o}}_{\text{6}}}{{\text{O}}_{\text{18}}})]\) were described as building blocks in the structure of (NH4)28Pr8Mo58O200·40H2O [9].
The protonation of Pt–O–Mo bridging oxide ligands results in the formation of proton-bound dimers (for example, [(PtMo6O24)2H7]9– and [(PtMo6O24)2H9]7– [7]) or even [(PtMo6O24)3Hx]n– polymer structures [10]. Thus, a change in pH can be used in searching for new types of the supramolecular organization in the mentioned systems. However, all these studies were carried out on molybdenum complexes. There are no data about similar behavior of tungsten derivatives in the literature. In this work, the protonation of the Pt–O–W coordinate with subsequent dimerization is reported for the first time for [PtIVW6O24]8–.
EXPERIMENTAL
Na5[H3PtW6O24]·20H2O was synthesized according to the procedure [11]. IR spectra were recorded using a diamond attachment on a FT-801 spectophotometer (Simex, Russia). TGA was carried out on a NETZSCH TG 209 F3 device in aluminum crucibles on heating the samples from 30 °C to 300 °C with a step of 10 °C. EDRS was performed on a JSM 6700F scanning electron microscope with an EDS EX-23000 BU attachment.
Synthesis of Cs8.1Na2.9[H5(PtW6O24)2]·8H2O (1). Na5H3[PtW6O24]·20H2O (0.428 g, 0.2 mmol) was dissolved in 8 mL of H2O; 200 µL of 1М HNO3 were added, and slightly heated (50 °C). Cesium sulfate (0.574 mg, 1.6 mmol) was added to the solution and stirred until dissolution. Yellow needle-shaped crystals of a product formed within a day. Additional portions of crystals can be obtained from the mother liquid when cooled in a refrigerator (5 °C). Yield: 0.405 g (87.8%).
IR (ATR, cm–1): 1088 w, 925 w, 909 w, 856 m, 802 m, 660 vs, 652 vs, 637 s, 622 s, 609 s, 595 s, 585 s (Fig. S3, Supplementary Materials).
EDRS: found Cs, Na, Pt, W (%): 23.2, 1.4, 8.3, 47.6; calculated Cs, Na, Pt, W (%): 23.1, 1.4, 8.4, 47.4.
TGA: found 3.2% H2O; calculated 3.1%. (Fig. S1, Supplementary Materials).
Single crystal X-ray diffraction (XRD). The structure of complex 1 was determined following the standard procedure on a Bruker D8 Venture diffractometer at 150 K with MoKα (λ = 0.71073 Å). The reflection intensities were measured by ω- and φ-scanning of narrow (0.5°) frames. Absorption correction was applied empirically using SADABS [12]. The structures were solved empirically using SHELXT [13] and refined by full-matrix LSM in the anisotropic approximation for non-hydrogen atoms using the SHELXL 2017\1 algorithm [14] in the ShelXle program [15]. Crystallographic data and structure refinement results are given in Table 1. Hydrogen atoms of solvate water molecules and protons on the polyoxoanion surface were not located.
The atomic coordinates and other structural parameters of complex 1 have been deposited with the Cambridge Crystallographic Data Center (No. 2167933; deposit@ccdc.cam.ac.uk or http://www.ccdc.cam. ac.uk/data_request/cif).
Powder XRD. The powder XRD study of polycrystalline samples was carried out on a Shimadzu XRD 7000 diffractometer with a linear Dectris Mythen2 detector at room temperature and CuKα radiation selected with a Ni filter.
RESULTS AND DISCUSSION
When a cesium sulfate solution is added to an aqueous solution of Na5[H3PtW6O24]·20H2O, a slow formation of yellow needle-shaped crystals of the Cs8.1Na2.9[(PtW6O24)2H5]·8H2O complex occurs. The crystalline product was characterized by single crystal XRD. A dry freshly-prepared sample has a high phase frequency (Fig. S2, Supplementary Materials). However, a long-term storage (for several months) leads to a loss of crystallization water molecules. The complex does not exhibit significant solubility in water even upon boiling and is stable in 1М sulfuric acid.
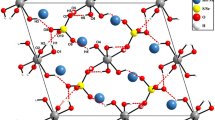
Compound 1 crystallizes in the monoclinic crystal system (symmetry space group C2/c) with unit cell parameters a = 20.8524(11) Å, b = 14.3943(8) Å, c = 20.7951(12) Å, β = 102.307(2)°. The main building block of the crystal structure is the proton-bound [(PtW6O24)2H5]11– dimer (Fig. 2). The asymmetric unit of the unit cell contains only one [PtIVW6O24]8– anion; correspondingly, the inversion center is in the geometric center of the dimer. The main geometric characteristics of the anion are listed in Table 2. These data show a minor distortion of the {PtO6} octahedron with bond lengths being in a range of 1.999(13)-2.031(15) Å. The coordination sphere of each tungsten atom includes three types of oxide ligands which noticeably differ in the W–O bond lengths: for W=O the respective distances are in a range of 1.696(15)-1.773(14) Å; d(W–μ2-O) = 1.901(16)-1.984(16) Å; d(W–μ3-O) = 2.095(13)-2.305(12) Å.
The interdimer space is filled with solvated cations and crystallization water molecules. The Cs and Na cation positions are mainly disordered (except for Cs1) and overlap with each other in a chaotic manner, which indicates the possibility to obtain solid solutions in the cation sublattice with a rather wide substitution interval. As a result of the structure refinement by LSM, 8.1 Cs and 2.9 Na per formula unit were found for a specific crystal. Two most ordered Na+ cation positions (50% occupancy) are very close to the central part of the dimer (Fig. 2). This indicates the size correspondence of the vacancy between two [PtIVW6O24]8– anions and the Na+ coordination sphere. In turn, this indicates the difficulty of complete substitution of all sodium cations in the crystal structure of this type.
Within the [(PtW6O24)2H5]11– dimer, five shortest distances between the oxide ligands of the neighboring anions can be distinguished, which correspond to five strong hydrogen bonds (Fig. 3). Four such contacts link the bridging oxide ligand in Pt–O–W and the terminal oxide ligand in W=O (two contacts at an O…O distance of 2.545 Å and two contacts at an O…O distance of 2.539 Å), uniquely indicating the proton position. The fifth contact implies the hydrogen bond between two μ3-O ligands at a distance of 2.635 Å. This means that the proton is disordered over two positions with an equal probability. Strong cation disorder suggests the existence of defects in the cation sublattice. In this case, there should be another proton on the POM surface for charge compensation. There are two short contacts (between the bridging oxide ligands and the crystallization water molecules), which can be suitable for this. The first one is between μ2-O(W–O–W) and a water molecule (d(O17–O6W) = 2.644 Å); the second is between μ3-O(Pt–O–W) and a water molecule (d(O21–O5W) = 2.707 Å). Given that the oxide ligands bonded with platinum are protonated first, it is O21 that should be protonated. However, the occupancy of both water positions (O5W and O6W) in the structure is 40%, which does not give an unambiguous answer about the presence of defects.
In the crystal structure, [(PtW6O24)2H5]11– form pseudo-layers of the AAA… topology (Fig. 4) oriented in the [110] plane. The position of dimers within a layer is shown in Fig. 5.
It is noteworthy that in the structure of initial Na5[H3PtW6O24]·20H2O there are only monomeric [H3PtW6O24]5– anions [10] in which the protons are most likely located on the oxide ligands of Pt–O–W coordinates. Thus, in our work, the proton-bound [(PtW6O24)2H5]11– dimeric anion is structurally described for the first time. This opens new prospects in the development of the chemistry of these compounds.
ACKNOWLEDGMENTS
The authors are grateful to the Ministry of Science and Higher Education of the Russian Federation for the possibility to carry out the sample characterization using the facilities of the Multi-Access Center of the Institute of Inorganic Chemistry, Siberian Branch, Russian Academy of Sciences.
REFERENCES
A. Wang, J. Li, and T. Zhang. Nat. Rev. Chem., 2018, 2, 65-81. https://doi.org/10.1038/s41570-018-0010-1
L. Zhang, Y. Ren, W. Liu, A. Wang, and T. Znahg. Natl. Sci. Rev., 2018, 5, 653-672. https://doi.org/10.1093/nsr/nwy077
N. V. Izarova, M. T. Pope, and U. Kortz. Angew. Chem., Int. Ed., 2012, 51, 9492-9510. https://doi.org/10.1002/anie.201202750
P. Putaj and F. Lefebvre. Coord. Chem. Rev., 2011, 255, 1642-1685. https://doi.org/10.1016/j.ccr.2011.01.030
A. V. Anyushin, P. A. Abramov, A. L. Gushchin, C. Vincent, and M. N. Sokolov. Russ. J. Inorg. Chem., 2017, 62, 397-403. https://doi.org/10.1134/S0036023617040027
A. A. Mukhacheva, V. V. Volchek, V. V. Yanshole, N. B. Kompankov, A. L. Gushchin, E. Benassi, P. A. Abramov, and M. N. Sokolov. Inorg. Chem., 2020, 59, 2116-2120. https://doi.org/10.1021/acs.inorgchem.9b02898
U. Lee and Y. Sasaki. Chem. Lett., 1984, 13, 1297-1300. https://doi.org/10.1246/cl.1984.1297
A. Ogawa, H. Yamato, U. Lee, H. Ichida, A. Kobayashi, and Y. Sasaki. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 1879-1881. https://doi.org/10.1107/S0108270188007073
A. M. Fedoseev, M. S. Grigorev, A. I. Yanovskii, Y. T. Struchkov, and V. I. Spitsyn. Dokl. Phys. Chem., 1987, 297, 477-479.
V. W. Day, J. C. Goloboy, and W. G. Klemperer. Eur. J. Inorg. Chem., 2009, 2009, 5079-5087. https://doi.org/10.1002/ejic.200900873
U. Lee, A. Kobayashi, and Y. Sasaki. Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1983, 39, 817-819. https://doi.org/10.1107/S0108270183006460
G. M. Sheldrick. SADABS: Program for scaling and correction of area detector data. Germany, Göttingen: University of Göttingen, 1996.
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3-8. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3-8. https://doi.org/10.1107/S2053229614024218
C. B. Hübschle, G. M. Sheldrick, and B. Dittrich. J. Appl. Crystallogr., 2011, 44, 1281-1284. https://doi.org/10.1107/S0021889811043202
Funding
The work was supported by the Russian Science Foundation (grant No. 19-73-10027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 9, 97978.https://doi.org/10.26902/JSC_id97978
Supplementary material
Rights and permissions
About this article
Cite this article
Kuznetsova, A.A., Abramov, P.A. & Sokolov, M.N. STRUCTURAL FEATURES OF THE [(PtW6O24)2H5]11– PROTON-BOUND DIMER. J Struct Chem 63, 1484–1490 (2022). https://doi.org/10.1134/S0022476622090098
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622090098