Abstract
The crystal structures of 3-chloro-5-hydroxy-1-(4-methylbenzyl)-4-[4-phenyl-1H-1,2,3-triazole-1-yl]-1,5-dihydro-2Н-pyrrol-2-one rac-1 and 1-benzyl-3-chloro-5-hydroxy-4-[4-phenyl-1H-1,2,3-triazole-1-yl]-1,5-dihydro-2Н-pyrrol-2-one rac-2 are studied. The reproducibility of a same-type homochiral chain in both chiral and racemic crystals is found. Molecular conformations, secondary interactions additionally cross-linking the chain, and π…π contacts between the cyclic molecular moieties are analyzed.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The production of pure enantiomers is becoming increasingly important not only for pharmaceutical industry, but also for agrochemistry and bioengineering [1-4]. Currently, there is clear evidence that often only one enantiomer of a chiral drug provides the desired physiological effect. In many cases, another enantiomer has no effect or even harmful [5-7].
Direct enantiomer crystallization from a racemic solution is possible only when enantiomers in their mixtures form separate optically pure crystals, and thus, the respective racemates are conglomerates, i.e., mixtures of crystals of both enantiomers. This phenomenon was called the spontaneous resolution of enantiomers. However, only 5% to 10% chiral substances are conglomerates [8, 9]. This indicates that during the formation of crystalline racemates heterochiral interactions are predominant and easier than homochiral [10].
The spontaneous resolution was first described by Louis Pasteur in 1848, when he manually separated left-handed crystals of sodium ammonium tartrate from right-handed ones [11]. The spontaneous chiral resolution during crystallization in the absence of any chiral source is of considerable interest in the context of homochirality as such [12-15] and absolute asymmetric synthesis in the solid state [16-19].
A necessary but not always sufficient condition for the spontaneous enantiomer resolution is the formation of homochiral associates [20-24]. Previously, we noted the fact of the stable reproducibility of homochiral hydrogen-bonded chains in a series of 4-arylsulfonyl-2(5Н)-furanones regardless of whether in a chiral or racemic crystal they occur [25]. The fact of the reproducibility of chemically and stereochemically identical homochiral chains in the crystals of two conglomerates, which adopts different symmetries depending on the crystal system (a zigzag chain along the twofold axis in the monoclinic conglomerate crystal and a sixth-order spiral in the hexagonal conglomerate crystal) was mentioned in [26, 27].
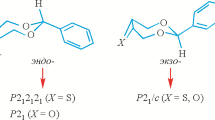
In this work, we performed the comparative analysis of the crystal structures of two representatives of N-substituted 3-pyrroline-2-ones (Scheme 1) in terms of the occurrence of different supramolecular associates, inter- and intramolecular interactions. The crystallo- and stereochemical interest was caused by the reproducibility of a same-type homochiral chain in both chiral and racemic crystals despite a change in the molecular conformation and the type of secondary interactions, additionally cross-linking the chain. A detailed consideration of the features of the homochiral chain reproduction in homochiral and racemic crystals is the subject of this article.
EXPERIMENTAL
Synthesis of compounds. 3-Chloro-5-hydroxy-1-(4-methylbenzyl)-4-[4-phenyl-1H-1,2,3-triazole-1-yl]-1,5-dihydro-2Н-pyrrol-2-one rac-1 and 1-benzyl-3-chloro-5-hydroxy-4-[4-phenyl-1H-1,2,3-triazole-1-yl]-1,5-dihydro-2Н-pyrrol-2-one rac-2 were synthesized according to the procedure previously described in [28].
Single crystal X-ray diffraction (XRD) experiments were carried out on a Rigaku XtaLab Synergy S diffractometer [λ(CuKα) = 1.54184 Å] at 100(2) K. The data were collected and edited, and the unit cell parameters were refined with the CrysAlisPro program; the absorption correction was applied using the ABSPACK program. The structures were solved by a direct method using SHELXT [29] and refined by the least squares method first in the isotropic and then in the anisotropic approximation (for all non-hydrogen atoms) using the SHELXL programs [30] within the Olex2 software [31]. The coordinates of hydrogen atoms were calculated based on stereochemical criteria and refined with the corresponding riding models. The intermolecular interactions were analyzed, and the figures were drawn using the PLATON [32] and Mercury [33] programs. The crystallographic data for the structures of 1a, 1b, and 2 reported in this article have been deposited with the Cambridge Crystallographic Data Center and are freely available on request on the website www.ccdc.cam.ac.uk/data_request/cif. The most important characteristics of the experiments, structure refinement, and the reference numbers are given in Table 1.
Powder XRD. The XRD experiments were performed on a Bruker D8 Advance X-ray diffractometer with a Vario attachment and a Vantec linear coordinate detector. CuKα1 radiation (1.54063 Å) monochromated with a curved Johansson monochromator was used. The X-ray tube operating mode of 40 kV and 40 mA was used. The experiments were carried out at room temperature in the Bragg–Brentano geometry with a flat sample. Powder samples were deposited on a surface of a single-crystal silicon plate with the minimum background scattering. Powder XRD patterns were recorded in the 2θ scattering angle range of 2-80°, step of 0.008°, point acquisition time of 0.1-1.0 s. Several XRD patterns in different experimental modes and with different acquisition times were measured for the sample; their comparison allowed us to control the sample stability over time. Since the experimental results showed the sample stability and the invariance of the XRD patterns, the series of the XRD patterns of the sample were summarized.
DSC thermograms were measured on a Netzsch DSC 204 F1 Phoenix device (τ-sensor) in aluminum crucibles at a scanning speed of 5 K/min. The sample weights were about 1-2 mg. The samples were weighted on Sartorius CPA 2P scales. Calibration of the heat flux and the temperature scale was controlled using indium and naphthalene samples as the references.
RESULTS AND DISCUSSION
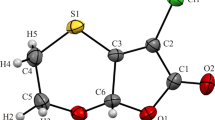
Structural formulas of the compounds and the partial numeration are given in Scheme 1. Note that the C5 atom is the only asymmetric center in the molecules.
For compound 1 two single crystals with slight distinctions in cut were isolated from the mixture (Fig. 1).
The structure of the crystal of 1a was solved in the chiral monoclinic space group P21. The asymmetric unit of the unit cell is represented by a single molecule (Z = 2, Z′ = 1). The studied single crystal is formed by the (S)-enantiomer; the sample as a whole is a racemic conglomerate. The structure of the 1b crystal was solved in the monoclinic system in the space group C2/c. The asymmetric unit of the unit cell contains one molecule (Z = 8, Z′ = 1); the sample is a racemic compound.
Consider the molecular conformation in the 1a and 1b crystals. As for the substituents at the central molecular moiety, the pyrrolinone ring, the para-tolyl moiety and the hydroxyl group are located on one side in the 1a crystal (Fig. 2a) and on opposite sides in the 1b crystal (Fig. 2b).
The general molecular conformation can be represented by a set of torsion angles along single bonds between the aromatic moieties (Table 2). Note that in the 1a crystal, the molecule is significantly flattened in the moiety containing pyrrolinone and triazole heterocycles, along with the phenyl ring linked with the latter (the respective torsion angles along single bonds between them have small values). At the same time, the benzyl moiety is rotated relative to the pyrrolinone ring in an unexpected way: in the N1–C6–C7–C8 moiety there is a completely eclipsed conformation, which we have not observed before for compounds with the similar structural moiety [26, 27, 34-36].
In the 1b crystal, the moiety containing pyrrolinone and triazole rings, and the phenyl ring adjacent to it is non-planar, which is reflected by large values of the key torsion angles. Note also a fundamentally different turn of the para-tolyl moiety relative to the central heterocycle: there is a skewed conformation along the C6–C7 and N1–C6 bonds.
In the 1a crystal, through the intermolecular O5–H5⋯N4′ hydrogen bond a homochiral hydrogen-bonded chain is formed along the 0b axis (by the screw twofold axis Fig. 3). Interestingly that this hydrogen-bonded chain is characterized by a number of additional secondary interactions, among which both secondary 1,2-cross-linking (occurring between the adjacent molecules) and 1,3-cross-linking (occurring through the next nearest molecule; i.e., between the molecules symmetrically related by a simple translation operation) can be distinguished. The contacts also differ by the type of interactions. For example, within the homochiral hydrogen-bonded chain in the 1a crystal there are 1,2-secondary O5–H5⋯N3′ and C5–H5A⋯N3′ interactions. The simultaneous occurrence of these interactions and a classical hydrogen bond is possible due to the mutual almost perpendicular arrangement of the adjacent molecules in the chain. The geometric parameters of the classical and non-classical hydrogen bonds are given in Table 3.
Along with this, the homochiral chain in the 1a crystal is also characterized by secondary 1,3- cross-linking due to the interactions of π-systems of the aromatic triazole and phenyl rings and the conjugated moiety of the pyrrolinone heterocycle (Fig. 4, Table 4).
Despite that the molecular conformation substantially differs in the 1b crystal from the concentration in the 1a crystal and the racemic composition of the 1b crystal, there are similar homochiral chains due to the hydrogen bonding of the hydroxyl hydrogen atom and the nitrogen atom of the triazole system (Fig. 5). Therewith, molecules in the chain have different mutual orientations, which results in a fundamentally different secondary 1,2-cross-linking: namely, through two C–H⋯O interactions involving the hydroxyl oxygen atom and hydrogen atoms of the phenyl and triazole rings. The parameters of these interactions are given in Table 3.
In the crystal of racemic compound 1b, unlike the chiral 1a crystal, π…π interactions cross-link the adjacent chains of both the same and opposite chirality rather than occur within the hydrogen-bonded chain (Fig. 6). The geometric parameters of these π…π contacts are given in Table 4.
To find out which phase is more preferable thermodynamically, we vigorously stirred the suspension of the initial sample of 1 in acetonitrile for a long time at room temperature [26, 37]. According to the powder XRD data, the sample thus obtained is represented by pure racemic conglomerate 1a, which indicates its thermodynamic stability (Fig. 7).
In order to obtain information on the thermal behavior of compound 1 the DSC method was used. The thermogram of the suspended sample demonstrates a narrow endothermic peak at 214 °C corresponding to melting of this form with the exothermic peak of the thermal destruction of the substance, immediately following it (Fig. 8). Previously, we have obtained metastable polymorphs of compounds and studied their thermochemical properties during melt crystallization [38-40], but for compound 1 it was not possible to avoid the melt decomposition and to carry out its crystallization, even at high scanning speeds and a transition to the cooling cycle before the melting was completed.
The rac-2 compound differs from the previous one by the absence of the methyl group in a para-position of the aryl ring at the nitrogen atom. The 2 crystal is isostructural with the 1a crystal; its structure was also solved in the chiral monoclinic space group P21 with very similar unit cell parameters; the asymmetric unit of the unit cell is represented by a single molecule (Z = 2, Z′ = 1). Correspondingly, the molecular conformation is the same as in the 1a crystal (Fig. 9). The studied single crystal is formed by the (S)-enantiomer. Thus, the sample is a racemic conglomerate.
In the crystal of racemic conglomerate 2, a homochiral chain, similar to that in the 1a crystal, is formed through O–H⋯N hydrogen bonds along the 0b axis, which is characterized by similar secondary 1,2-cross-linking through O–H⋯N and C–H⋯O interactions (Table 3). In the composition of the homochiral chain, two π…π (conventionally, between the first and the third molecules) interactions were found, which took place due to the mutual parallel arrangement of these molecules in the chain thereby performing secondary 1,3-cross-linking (Table 4).
In the crystals of racemic conglomerates 1a and 2, intermolecular interactions between the Cl1 and O2 atoms cross-link the adjacent hydrogen-bonded chains (Fig. 10, Table 5).
CONCLUSIONS
The reproducibility of the homochiral hydrogen bonding is found in 3-chloro-5-hydroxy-1-(4-methylbenzyl)-4-[4-phenyl-1H-1,2,3-triazole-1-yl]-1,5-dihydro-2Н-pyrrol-2-one conglomerate and racemic compound crystals, which occurs despite different conformations of the key molecule, and different types of secondary interactions additionally cross-linking the same-type homochiral chains.
REFERENCES
H. Lorenz and A. Seidel-Morgenstern. Angew. Chem., Int. Ed., 2014, 53, 1218. https://doi.org/10.1002/anie.201302823
A. M. Rouhi. Chem. Eng. News, 2003, 81, 56. https://doi.org/10.1021/cen-v081n011.p056
R. A. Sheldon. Chirotechnology: Industrial Synthesis of Optically Active Compounds. New York: Marcel Dekker, 1993.
G. Q. Lin, Q. D. You, and J. F. Cheng. Chiral Drugs: Chemistry and Biological Action. Hoboken: Wiley, 2011.
S. C. Stinson. Chem. Eng. News, 2001, 79, 45. https://doi.org/10.1021/cen-v079n032.p045
A. M. Rouhi. Chem. Eng. News, 2003, 81, 45. https://doi.org/10.1021/cen-v081n004.p045
J. Blumenstein. In: Chirality in Industry II: Developments in the Manufacture and Applications of Optically Active Compounds / Eds. A.N. Collins, G.N. Sheldrake, J. Crosby. Chichester: Wiley, 1997, 11-18.
I. Katsuki, Y. Motoda, Y. Sunatsuki, N. Matsumoto, T. Nakashima, and M. Kojima. J. Am. Chem. Soc., 2002, 124, 629. https://doi.org/10.1021/ja0123960
A. Collet. Enantiomer, 1999, 4, 157.
C. P. Brock, W. B. Schweizer, and J. D. Dunitz. J. Am. Chem. Soc., 1991, 113, 9811. https://doi.org/10.1021/ja00026a015
L. Pasteur. Ann. Chim. Phys., 1848, 24, 442.
M. D. Cohen and G. M. J. Schmidt. J. Chem. Soc., 1964, 1996. https://doi.org/10.1039/jr9640001996
B. S. Green, M. Lahav, and D. Rabinovich. Acc. Chem. Res., 1979, 12, 191. https://doi.org/10.1021/ar50138a001
S. F. Mason. Nature, 1984, 311, 19. https://doi.org/10.1038/311019a0
G. Kaupp and M. Haak. Angew. Chem., 1993, 105, 727. https://doi.org/10.1002/ange.19931050508
F. Toda, M. Yagi, and S. Soda. J. Chem. Soc. Chem. Commun., 1987, 1413. https://doi.org/10.1039/C39870001413
M. Takahashi, N. Sekine, T. Fujita, S. Watanabe, K. Yamaguchi, and M. Sakamoto. J. Am. Chem. Soc., 1998, 120, 12770. https://doi.org/10.1021/ja982696q
S. Kohmoto, H. Masu, C. Tatsuno, K. Kishikawa, M. Yamamoto, and K. Yamaguchi. J. Chem. Soc., Perkin Trans. 1, 2000, 4464. https://doi.org/10.1039/b005142j
M. Sakamoto, T. Iwamoto, N. Nono, M. Ando, W. Arai, T. Mino, and T. Fujita. J. Org. Chem., 2003, 68, 942. https://doi.org/10.1021/jo0266689
P. A. Levkin, Yu. A. Strelenko, K. A. Lyssenko, V. Schurig, and R. G. Kostyanovsky. Tetrahedron: Asymmetry, 2003, 14, 2059. https://doi.org/10.1016/S0957-4166(03)00399-9
P. A. Levkin, K. A. Lyssenko, V. Schurig, and R. G. Kostyanovsky. Mendeleev Commun., 2003, 13, 106. https://doi.org/10.1070/MC2003v013n03ABEH001747
A. N. Kravchenko, G. K. Kadorkina, A. S. Sigachev, E. Yu. Maksareva, K. A. Lyssenko, P. A. Belyakov, O. V. Lebedev, O. N. Kharybin, N. N. Makhova, and R. G. Kostyanovsky. Mendeleev Commun., 2003, 13, 114. https://doi.org/10.1070/MC2003v013n03ABEH001736
R. G. Kostyanovsky, V. Schurig, O. Trapp, K. A. Lyssenko, B. B. Averkiev, G. K. Kadorkina, A. V. Prosyanik, and V. R. Kostyanovsky. Mendeleev Commun., 2002, 12, 137. https://doi.org/10.1070/MC2002v012n04ABEH001612
R. G. Kostyanovsky, I. A. Bronzova, and K. A. Lyssenko. Mendeleev Commun., 2002, 12, 4. https://doi.org/10.1070/MC2002v012n01ABEH001516
O. A. Lodochnikova, Yu. K. Voronina, L. Z. Latypova, D. B. Krivolapov, A. R. Kurbangalieva, and I. A. Litvinov. Russ. Chem. Bull., 2013, 62, 1218. https://doi.org/10.1007/s11172-013-0167-1
O. A. Lodochnikova, A. R. Zaripova, R. R. Fayzullin, A. I. Samigullina, I. I. Vandyukova, L. N. Potapova, and A. R. Kurbangalieva. CrystEngComm, 2018, 20, 3218. https://doi.org/10.1039/C8CE00369F
D. P. Gerasimova, A. F. Saifina, D. V. Zakharychev, R. R. Fayzullin, A. R. Kurbangalieva, and O. A. Lodochnikova. CrystEngComm, 2021, 23, 3907. https://doi.org/10.1039/D1CE00227A
E. Sh. Saigitbatalova, K. R. Ramazanova, I. D. Shutilov, T. A. Gazizov, D. R. Islamov, and A. R. Kurbangalieva. Materialy Mezhdunarodnoi nauchnoi konferentsii studentov, aspirantov i molodykh uchenykh “Lomonosov-2021” (Abstracts of the International Scientific Conference for Undergraduate and Graduate Students and Young Scientists “Lomonosov-2021”), Moscow, Russia, April 12-23. Moscow: Pero, 2021, 702. [In Russian]
G. M. Sheldrick. Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3. https://doi.org/10.1107/S2053273314026370
G. M. Sheldrick. Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3. https://doi.org/10.1107/S2053229614024218
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. J. Puschmann. Appl. Crystallogr., 2009, 42, 339. https://doi.org/10.1107/S0021889808042726
A. L. Spek. Acta Crystallogr., Sect D: Biol. Crystallogr., 2009, 65, 148. https://doi.org/10.1107/S090744490804362X
C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler, and P. A. Wood. J. Appl. Crystallogr., 2020, 53, 226. https://doi.org/10.1107/S1600576719014092
O. A. Lodochnikova, L. S. Kosolapova, A. F. Saifina, A. T. Gubaidullin, R. R. Fayzullin, A. R. Khamatgalimov, I. A. Litvinov, and A. R. Kurbangalieva. CrystEngComm, 2017, 19, 7277. https://doi.org/10.1039/C7CE01717K
D. P. Gerasimova, A. F. Saifina, D. V. Zakharychev, I. I. Vandyukova, R. R. Fayzullin, A. R. Kurbangalieva, and O. A. Lodochnikova. J. Struct. Chem., 2020, 61(3), 476. https://doi.org/10.1134/S0022476620030142
D. P. Gerasimova, A. F. Saifina, D. V. Zakharychev, A. R. Zaripova, R. R. Fayzullin, A. R. Kurbangalieva, and O. A. Lodochnikova. J. Struct. Chem., 2021, 62(5), 727. https://doi.org/10.1134/S0022476621050097
R. R. Fayzullin., D. V. Zakharychev, A. T. Gubaidullin, O. A. Antonovich, D. B. Krivolapov, Z. A. Bredikhina, and A. A. Bredikhin. Cryst. Growth Des., 2017, 17, 271. https://doi.org/10.1021/acs.cgd.6b01522
A. A. Bredikhin, D. V. Zakharychev, Z. A. Bredikhina, A. V. Kurenkov, D. B. Krivolapov, and A. T. Gubaidullin. Cryst. Growth Des., 2017, 17, 4196. https://doi.org/10.1021/acs.cgd.7b00510
A. A. Bredikhin, D. V. Zakharychev, A. T. Gubaidullin, R. R. Fayzullin, A. I. Samigullina, and Z. A. Bredikhina. Cryst. Growth Des., 2018, 18, 3980. https://doi.org/10.1021/acs.cgd.8b00321
A. A. Bredikhin, D. V. Zakharychev, A. T. Gubaidullin A. I. Samigullina, and Z. A. Bredikhina. Cryst. Growth Des., 2021, 21, 3111. https://doi.org/10.1021/acs.cgd.0c01570
Funding
The work was supported by the State Assignment of the Federal Research Center “Kazan Scientific Center”, Russian Academy of Sciences.
The synthesis of the key compound was supported by the Strategic Academic Leadership Program of the Kazan (Privolzhsky) Federal University (Priority-2030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 9, 97832.https://doi.org/10.26902/JSC_id97832
Rights and permissions
About this article
Cite this article
Gerasimova, D.P., Saigitbatalova, E.S., Islamov, D.R. et al. REPRODUCIBILITY OF A HOMOCHIRAL HYDROGEN-BONDED CHAIN IN CONGLOMERATE AND RACEMIC COMPOUND CRYSTALS OF THE TRIAZOLE DERIVATIVE OF 3-PYRROLINE-2-ONE. J Struct Chem 63, 1434–1445 (2022). https://doi.org/10.1134/S0022476622090062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622090062