Abstract
Oxidative stress induced by harmful substances activates inflammatory signaling pathways and causes excessive proliferation of keratinocytes, which is related to the occurrence of psoriasis. Rutin, a natural citrus flavonoid glycoside, exhibits protective effects against oxidative stress. However, whether rutin is able to influence oxidative stress in keratinocytes remains unclear. In the present study, an in vitro cell model of human keratinocytes (HaCaT) treated with H2O2 was used to explore whether rutin can prevent oxidative stress. The present findings suggest that rutin protected HaCaT cells against oxidative damage by inhibiting ROS, NO and MDA secretion, increasing SOD activity and restoring GSH-Px activity. In addition, rutin supplement limited IL-6, IL-1β and IL-23A production in HaCaT cells. Moreover, rutin upregulated Nrf2 expression, and promoted the downstream NQO1 and HO-1 expression. These results suggest that rutin may inhibit HaCaT cell oxidative stress by modulating the Nrf2-regulated pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Psoriasis is clinically characterized by red patches of skin with a pronounced boundary, which are often covered with multiple silvery white scaly layers and accompanied by keratinocyte proliferation, obvious itching and scaling. Psoriasis is a common chronic immune-related inflammatory disease that mainly affects the skin and joints and is mediated by overactive Th17 response [1, 2]. Several studies have reported that oxidative stress plays an important role in the development and progression of various skin diseases, such as psoriasis [3–5], and the role of reactive oxygen species (ROS) in psoriasis has been demonstrated [6]. Oxidative stress is often related to exogenous factors such as environmental pollution, ultraviolet rays and chemical substances, leading to inflammatory reactions and tissue damage, which subsequently promote the generation of endogenous oxidants [7].
Keratinocytes are a major component of the epidermis [8]. Oxidative stress-induced ROS and reactive nitrogen species (RNS) can activate inflammatory signaling pathways and cause excessive proliferation of keratinocytes [9]. Therefore, the oxidative stress is closely related to the occurrence and development of the disease, and drug intervention against such pathophysiological phenomena may be a new target for the treatment of psoriasis.
Rutin is a common dietary flavonoid glycoside widely found in vegetables and citrus fruits that has a variety of regulatory effects on cell functions, including antioxidative, anti-inflammatory, antiallergic, antiviral, anticancer, and antiangiogenic effects [10]. Previous study has shown that rutin is capable of reducing the secretion of important mediators of inflammatory and immune responses such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), promote the migration of white blood cells, and increase immunoglobulin levels in the body [11]. Furthermore, rutin effectively protects skin fibroblasts and keratinocytes from oxidative stress following UV exposure [12, 13]. The positive contributions of rutin in aging and cardiovascular diseases have been shown in previous studies [14, 15]. However, the effectiveness of rutin in the treatment of psoriasis remains to be further clarified.
In this study, human immortalized keratinocytes (HaCaT) treated with H2O2 were used to determine the effects of rutin in vitro. Moreover, we also investigated the important factors related to oxidative stress and inflammation to further explore the possible regulatory mechanisms of rutin in psoriasis.
MATERIALS AND METHODS
Cell culture
The HaCaT cell line (GAINING BIOLOGICAL, Shanghai, China) was cultured in high-glucose Dulbecco’s modified Eagle medium (DMEM, Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum (BioInd, Inc., Shanghai, China) and 1% penicillin/streptomycin (Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C and 5% CO2. Rutin (Solarbio Science & Technology Co., Ltd., Beijing, China) was prepared as a 20 mg/mL stock solution in phosphate buffered saline (containing 0.1% dimethyl sulfoxide, DMSO) and diluted in DMEM to the specified concentrations used in each experiment. H2O2 was obtained from Thermo Fisher Scientific, Inc. (Thermo Fisher Scientific, Inc.) and prepared as a 10 mM stock solution in DMEM. HaCaT cells in the control group were pretreated with DMSO, and cells in the experimental groups were pretreated with rutin for 1 hour before incubation with or without H2O2 (200 µM, diluted in DMEM) for 24 hours as described before [16].
Cell viability assay
A cell viability CCK-8 assay was performed to measure the effect of rutin and H2O2 on HaCaT cell viability. In this assay the water-soluble tetrazolium salt in the reagent can be reduced by dehydrogenase in cell mitochondria and thereby converted to formazan dye, which has an absorbance at 450 nm, and the optical density is positively related to the number of viable cells. HaCaT cells (4 × 103 cells/well) were cultured in 96-well plates and treated with rutin at various concentrations (0, 6.25, 12.5, 25, 50, 100 and 200 µg/mL) for 1 h. In addition, cells (4 × 103 cells/well) were cultured in 96-well plates and treated with H2O2 (0, 50, 100, 200, 300, 400, 500, 600, 700, and 800 µM) for 24 h. Subsequently, the effect of ruitn on the viability of HaCaT cells treated with H2O2 was studied, and the cells (4 × 103 cells/well) were first treated with rutin (0, 6.25, 12.5, 25, 50, 100 and 200 µg/mL) for 1 h followed by incubation with 200 µM H2O2 for 24 h. Cells then were treated using CCK-8 kits (Cat# CK04-11, Dojindo, Shanghai, China), and the optical density at 450 nm was measured using a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Measurement of oxidative stress factors
The content of intracellular ROS in HaCaT cells was detected by ROS assay kit (Cat# S0033M, Beyotime, Shanghai, China). For this, cells (4 × 104 cells/well) were seeded into 96-well plates and pretreated with rutin (100 µg/mL) for 1 hour, followed by incubation with or without H2O2 (200 µM) for 24 h. Cells then were washed with DMEM and incubated with 10 µL of dichloro-dihydro-fluorescein diacetate (DCFH-DA) (1 µM) at 37°C for 20 min in the dark, then ROS positive populations of HaCaT cells were measured by a flow cytometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 488 nm laser wavelength and 525 nm detection wavelength. The content of nitric oxide (NO) in HaCaT cells was determined in the following experiments using a commercial kit (Cat# A013-2-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). For this, cells (3 × 105 cells per mL) were seeded into 6-well plates and treated with rutin and H2O2 as described above. The absorbance of the reaction mixtures was measured at 540 nm with a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and NO content was calculated using the NaNO2 standard calibration curve. The malondialdehyde (MDA), total superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) content and total antioxidant capacity (T-AOC) in HaCaT cells were evaluated by the corresponding test kit (Cat# A003-1, A001-3, A005-1, A015-2-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturers’ instructions. For this, cells (2 × 106 cells/well) were seeded into 6-well plates and treated as mentioned above. MDA content was analyzed by the thiobarbituric acid (TBA) method. Briefly, MDA reacts with TBA to generate an MDA-TBA adduct whose absorbance can be measured at 532 nm. The activity of SOD was measured by the water-soluble tetrazolium salt (WST) method, 20 µL supernatant, 20 µL enzyme working solution and 200 µL substrate solutions were mixed and incubated at 37°C for 20 min. The absorbance was measured on a microplate reader at 450 nm. GSH-Px activity was measured using the GSH assay kit based on the manufacturer’s instruction. A series of enzymatic reactions was activated by GSH-Px in cells which subsequently led to the conversion of GSH to oxidized glutathione (GSSG). The change in absorbance during the conversion of GSH to GSSG was measured by microplate reader at 412 nm.
Protein content analysis
In all experiments after administration with rutin or H2O2 or both, cells were homogenized in PBS (Cat# G4202, Servicebio Technology Co., Ltd., Wuhan, China) at 4°C and centrifuged at 12,000 g for 5 min, the supernatant was collected and protein content was quantified using a bicinchoninic acid (BCA) protein assay kit (Cat# PC0020, Solarbio Science & Technology Co., Ltd., Beijing, China).
Enzyme-linked immunosorbent assay (ELISA)
HaCaT cells (1 × 106 cells/well) were cultured in 6-well plates and treated with rutin (100 µg/mL) for 1 hour, followed by incubation with or without H2O2 (200 µM) for 24 h, culture supernatants in each well were collected respectively and centrifuged at 3000 rpm for 20 min at 4 °C, then supernatants were harvested and analyzed for IL-6, IL-1β, and IL-23A in ELISA kits (MEIMIAN Co., Ltd., Jiangsu, China). The concentrations of the inflammatory factors in cell supernatants were determined according to the manufacturer’s protocols. The optical density (OD) of the samples after performing the tests was read at 450 nm by a microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Western blotting
HaCaT cells (1 × 106 cells/well) were cultured in 6-well plates and treated as mentioned above, then cells were washed three times with ice-cold PBS and lysed using 100 µL of RIPA buffer (Solarbio Science & Technology Co., Ltd., Beijing, China) containing a cocktail of protease inhibitors. Total proteins from HaCaT cells were extracted, and concentrations were determined by the BCA assay. 50 µg of protein was uploaded in each lane. Protein samples were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to a polyvinylidene difluoride membrane. The membranes were blocked in blocking buffer (Beyotime, Shanghai, China) for 2 h at room temperature and immunoblotted overnight at 4°C with the following primary antibodies: rabbit monoclonal anti-Nrf2 antibody (1 : 1000, Cat# ab62352), mouse monoclonal anti-Keap1 antibody (1 : 1000, Cat# ab119403), rabbit monoclonal anti-Heme Oxygenase 1 (HO-1) antibody (1 : 2000, Cat# ab52947), mouse monoclonal anti-NQO1 antibody (1 : 500, Cat# ab28947), rabbit monoclonal anti-GCLC antibody (1 : 1000, Cat# ab190685), and mouse monoclonal anti-β-actin antibody (1 : 5000, Cat# ab6276). The membrane was washed with washing buffer (Beyotime, Shanghai, China) and incubated with the corresponding secondary HRP-conjugated goat anti-rabbit antibody (1 : 2000, Cat# A0208, Beyotime) or goat anti-mouse antibody (1 : 2000, Cat# A0216, Beyotime) for 1 h at room temperature. Protein bands were visualized with enhanced chemiluminescence substrate (Beyotime) and quantified using FujiFilm Multi Gauge Ver. 3.0 software (Fuji Photo Film Co., Tokyo, Japan).
Statistical analysis
All data were analysed with GraphPad Prism software v5.01 (GraphPad Software Inc., San Diego, CA, USA) and are shown as the mean ± SEM. Comparisons between groups were analysed by one-way analysis of variance followed by Tukey’s multiple comparison test. p < 0.05 was considered as statistically significant.
RESULTS
Effects of rutin on H 2 O 2 -induced HaCaT cell viability
In this study, a commercial CCK-8 kit was used to test the viability of the cells using as a measured parameter the absorbance of formazan dye at 450 nm and assess the optimal concentration of H2O2 treatment. As shown in Fig. 1a, HaCaT cells exhibited approx. 80% viability after 24 h of incubation with 200 µM H2O2. Therefore, 200 µM H2O2 was employed to treat HaCaT cells in the following experiments. To assess the potential cytotoxicity induced by rutin treatment in HaCaT cells, cells were treated with various concentrations of rutin for 1 h, and cell viability was evaluated by CCK-8 assay. The results indicated that 200 µg/mL rutin administered for 1 h revealed significant toxicity. Thus, 100 µg/mL rutin or less was selected for the subsequent experiments (Fig. 1b). Next, the effect of rutin on the viability of HaCaT cells treated with H2O2 was studied. HaCaT cells were pretreated with rutin at 0, 6.25, 12.5, 25, 50, 100 and 200 µg/mL for 1 h followed by treatment with H2O2 (200 µM) for 24 h. As shown in Fig. 1c, 100 µg/mL rutin treatment successfully rescued H2O2-induced cell viability reduction, indicating a promising protective effect of rutin in HaCaT cell oxidative stress injury.
Rutin attenuated H 2 O 2 -induced oxidative stress in HaCaT cells
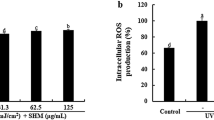
The increase of ROS and NO content are important causes of oxidative stress injury. Therefore, we investigated the capability of rutin to inhibit H2O2-induced ROS and NO production in HaCaT cells by using ROS assay kit and NO test kit respectively. The results in Figs. 2a–2b indicated that H2O2 treatment increased ROS and NO generation compared to the control group, which was significantly inhibited by 100 µg/mL rutin pretreatment. In addition, MDA levels were significantly enhanced following H2O2 treatment, and rutin was capable of antagonizing MDA elevation (Fig. 2c). Apart from ROS, NO and MDA, we also assessed the T-AOC, SOD and GSH-Px activities to evaluate the antioxidative properties of rutin. As shown in Figs. 2d–2f, 100 µg/mL rutin boosted the antioxidant capacity of HaCaT cells by increasing SOD activity and restoring GSH-Px activity reduced by H2O2.
Rutin inhibited cytokine production in H 2 O 2 -treated HaCaT cells
To validate the effect of rutin on the inflammatory response in H2O2-treated HaCaT cells. ELISA analysis was performed to measure the concentrations of IL-6, IL-1β and IL-23A in the cell supernatants. As shown in Fig. 3, H2O2 administration markedly enhanced IL-6, IL-1β and IL-23A content compared with the control group (Figs. 3a–3c). As expected, the alterations that occurred above were reversed by rutin pretreatment (Figs. 3a–3c).
Effect of rutin on the levels of Nrf2 and antioxidant system proteins in H 2 O 2 -induced HaCaT cells
Nrf2 is an important factor in the cellular antioxidant defense system. In the following experiment, the expression levels of Keap1 (kelch-like ECH-associated protein 1), Nrf2 (nuclear factor erythroid 2-related factor 2), NQO1 (NAD(P)H quinone oxidoreductase 1), HO-1 (heme oxygenase-1) and GCLC (glutamate-cysteine ligase catalytic subunit) were determined by Western blotting. The results showed that Nrf2 and NQO1 protein expression levels were decreased by H2O2 treatment, whereas rutin significantly reversed this downregulation in HaCaT cells (Figs. 4c, 4d). The protein expression of Keap1 and HO-1 showed an increasing or decreasing trend, but there was no statistically significant difference.
Effects of rutin on cell viability of HaCaT cells treated with H2O2. (a) Viability of HaCaT cells after incubation with H2O2 (0, 50, 100, 200, 300, 400, 500, 600, 700 and 800 µM) for 24 h. (b) Viability of HaCaT cells after treatment with rutin (0, 6.25, 12.5, 25, 50, 100 and 200 µg/mL) for 1 hour. (c) Effects of rutin pretreatment on the viability of HaCaT cells treated with 200 µM H2O2 for 24 h. The viability of HaCaT cells was assessed by the CCK8 kit, and absorbance was examined by a microplate reader at 450 nm. Data were analyzed by one-way analysis of variance followed by Tukey’s post-hoc test and expressed as the mean ± SEM. *—p < 0.05, **—p < 0.01, ***—p < 0.001, compared to the control group, #—p < 0.05, compared to the H2O2-treated group, n = 4.
Rutin attenuated H2O2-induced oxidative stress in HaCaT cells. HaCaT cells were treated with 100 µg/mL rutin for 1 h and further exposed to 200 µM H2O2 for 24 h. Oxidative stress biochemical indicators were measured by commercial kits. (a) ROS production, (b) NO levels, (c) MDA levels, (d) T-AOC, (e) SOD and (f) GSH-Px activity in HaCaT cells. Data are presented as the mean ± SEM. *—p < 0.05, **—p < 0.01, ***—p < 0.001, compared to the control group; #—p < 0.05, ##—p < 0.01, ###—p < 0.001, compared to the H2O2-treated group, n = 6.
Rutin inhibited cytokine production in H2O2-induced HaCaT cells. HaCaT cells were pretreated with 100 µg/mL rutin for 1 h followed by exposure to 200 µM H2O2 for 24 h. IL-6 (a), IL-1β (b), and IL-23A (c) content in cell supernatants were determined using commercial ELISA kits. The final absorbance values were measured at 450 nm using a plate reader. Data are presented as the mean ± SEM. *—p < 0.05, ***—p < 0.001, compared to the control group; ##—p < 0.01, ###—p < 0.001, compared to the H2O2-treated group, n = 5.
Effect of rutin on protein levels of Nrf2 and antioxidant system proteins in H2O2-induced HaCaT cells. HaCaT cells were treated with 100 µg/mL rutin for 1 h and further exposed to 200 µM H2O2 for 24 h. Total protein levels from HaCaT cells were determined by BCA assay, and protein expression was measured by Western blotting. (a) Representative Western blotting images. (b–f) Quantitative protein analysis of Keap1, Nrf2, NQO1, HO-1 and GCLC expression in HaCaT cells. Data are presented as the mean ± SEM. *—p < 0.05, **—p < 0.01, compared to the control group; ##—p < 0.01, compared to the H2O2-treated group, n = 3.
DISCUSSION
Increasing evidence shows that oxidative stress may aggravate the pathogenesis of psoriasis [4, 17]. Due to environmental problems such as heavy metal pollution, ionizing radiation, and ultraviolet radiation, the skin is constantly exposed to internal and external oxidizing conditions, which accelerate the production of ROS in the body [18]. Overgenerated ROS can upregulate the expression of proinflammatory cytokines and chemokines through signaling pathways such as MAPK/AP-1, NF-κB, and JAK-STAT or by increasing DNA modification and lipid peroxidation reaction products [19]. As a result, this causes the occurrence of psoriasis or leads to aggravation of the disease. Meanwhile, studies have shown that the antioxidant system in the skin lesions of patients with psoriasis is unbalanced, with reduced expression of antioxidant enzymes, which leads to weakened antioxidant capacity of cells [20]. The above findings have proven that oxidative stress damage is closely related to the occurrence of psoriasis. H2O2 is a reliable agent for modelling oxidative stress, and the in vitro oxidative stress model jointly constructed with HaCaT cells is more cost-effective and easy to reproduce and is suitable for the pathological study of a variety of oxidative stress-related skin diseases. In our study, to evaluate the sensitivity of HaCaT cells to oxidative stress, cells were treated with H2O2 [21].
The antioxidant protection system of the cell includes antioxidant molecules and antioxidant enzymes [22]. Under physiological conditions, the formation of peroxide and antioxidant substances is in a dynamic balance, which depends on the effectiveness of the elimination of ROS by antioxidant substances [23]. In addition, the contents of MDA and the activities of SOD and GSH-Px are usually used to provide a comprehensive assessment of the antioxidant capacity [24]. MDA is a final product of lipid peroxidation, which is the hallmark of ROS-induced injury and reflects the ROS content and cell injury [25]. In contrast to ROS, NO and MDA, GSH-Px and SOD are considered as the key antioxidant enzymes of the antioxidant defense system [26]. Studies have shown that rutin can activate antioxidant enzymes, including catalase, to minimize the formation of free radicals while inhibiting oxidative stress by enhancing the activities of antioxidant enzymes such as SOD, CAT, and GSH-Px [27, 28]. The present results revealed that the released ROS, NO and MDA of injured HaCaT cells increased and the antioxidant enzymes GSH-Px and SOD decreased, indicating that H2O2 induced cell oxidative damage in the model group. After pretreatment with rutin, the contents of ROS, NO and MDA declined significantly when compared with those in H2O2-treated HaCaT cells. The present investigation has demonstrated that rutin had a significant antioxidant capacity in vitro.
In addition, the pathogenesis of psoriasis is related to the excessive proliferation of epidermal keratinocytes and small proteins such as cytokines play an important role in this process. During the development of psoriasis, the increased expression of inflammatory cytokines such as TNF-α, IL-6, and IL-23 triggers inflammation and activates keratinocyte proliferation [29]. Our data showed that rutin inhibited H2O2-induced increases in IL-6, IL-1β and IL-23A levels, thus exerting an anti-inflammatory effect.
The key components of endogenous antioxidant systems are controlled by the transcription factor Nrf2 [30]. When oxidative stress occurs, Nrf2 translocates into the nucleus and heterodimerizes with small Maf proteins to transactivate genes containing antioxidant response element (ARE) and promote the transcription of genes involved in defenses against oxidative stress injury, such as HO-1, NQO1, and GCLC [31, 32]. As expected, our results suggested that rutin pretreatment up-regulates Nrf2 protein expression and promotes the downstream NQO1 expression.
In summary, our study demonstrates that rutin reduced the inflammatory response and enhances the antioxidant system in human keratinocytes via an Nrf2-dependent mechanism.
References
Benhadou F, Glitzner E, Brisebarre A, Swedlund B, Song Y, Dubois C, Rozzi M, Paulissen C, Del Marmol V, Sibilia M, Blanpain C (2020) Epidermal autonomous VEGFA/Flt1/Nrp1 functions mediate psoriasis-like disease. Sci Adv 6: eaax5849. https://doi.org/10.1126/sciadv.aax5849
Chang HW, Yan D, Singh R, Liu J, Lu X, Ucmak D, Lee K, Afifi L, Fadrosh D, Leech J, Vasquez KS, Lowe MM, Rosenblum MD, Scharschmidt TC, Lynch SV, Liao W (2018) Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome 6: 154. https://doi.org/10.1186/s40168-018-0533-1
Peluso I, Cavaliere A, Palmery M (2016) Plasma total antioxidant capacity and peroxidation biomarkers in psoriasis. J Biomed Sci 23: 52. https://doi.org/10.1186/s12929-016-0268-x
Wu L, Liu G, Wang W, Liu R, Liao L, Cheng N, Li W, Zhang W, Ding D (2020) Cyclodextrin-Modified CeO2 Nanoparticles as a Multifunctional Nanozyme for Combinational Therapy of Psoriasis. Int J Nanomedicine 15: 2515–2527. https://doi.org/10.2147/IJN.S246783
Agrawal YO, Mahajan UB., Mahajan HS, Ojha S (2020) Methotrexate-Loaded Nanostructured Lipid Carrier Gel Alleviates Imiquimod-Induced Psoriasis by Moderating Inflammation: Formulation, Optimization, Characterization, In-Vitro and In-Vivo Studies. Int J Nanomed 15: 4763–4778. https://doi.org/10.2147/IJN.S247007
Gunter NV, Teh SS, Lim YM, Mah SH (2020) Natural Xanthones and Skin Inflammatory Diseases: Multitargeting Mechanisms of Action and Potential Application. Front Pharmacol 11: 594202. https://doi.org/10.3389/fphar.2020.594202
Parvez S, Long M, Poganik JR, Aye Y (2018) Redox Signaling by Reactive Electrophiles and Oxidants. Chem Rev 118: 8798–8888. https://doi.org/10.1021/acs.chemrev.7b00698
Lehmann SG, Bourgoin-Voillard S, Seve M, Rachidi W (2017) Tubulin Beta-3 Chain as a New Candidate Protein Biomarker of Human Skin Aging: A Preliminary Study. Oxid Med Cell Longev 2017: 5140360. https://doi.org/10.1155/2017/5140360
Kim BH, Choi MS, Lee HG, Lee SH, Noh KH, Kwon S, Jeong AJ, Lee H, Yi EH, Park JY, Lee J, Joo EY, Ye SK (2015) Photoprotective Potential of Penta-O-Galloyl-β-DGlucose by Targeting NF-κB and MAPK Signaling in UVB Radiation-Induced Human Dermal Fibroblasts and Mouse Skin. Mol Cells 38: 982–990. https://doi.org/10.14348/molcells.2015.0169
Nafees S, Rashid S, Ali N, Hasan SK, Sultana S (2015) Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: role of NFκB/MAPK pathway. Chem-Biol Interact 231: 98–107. https://doi.org/10.1016/j.cbi.2015.02.021
Ganeshpurkar A, Saluja AK (2017) Protective effect of rutin on humoral and cell mediated immunity in rat model. Chem-Biol Interact 273: 154–159. https://doi.org/10.1016/j.cbi.2017.06.006
Gęgotek A, Bielawska K, Biernacki M, Dobrzyńska I, Skrzydlewska E (2017) Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol 12: 733–744. https://doi.org/10.1016/j.redox.2017.04.014
Gęgotek A, Ambrożewicz E, Jastrząb A, Jarocka-Karpowicz I, Skrzydlewska E (2019) Rutin and ascorbic acid cooperation in antioxidant and antiapoptotic effect on human skin keratinocytes and fibroblasts exposed to UVA and UVB radiation. Arch Dermatol Res 311: 203–219. https://doi.org/10.1007/s00403-019-01898-w
Choi SJ, Lee SN, Kim K, Joo d, Shin S, Lee J, Lee HK, Kim J, Kwon SB, Kim MJ, Ahn KJ, An IS, An S, Cha HJ (2016) Biological effects of rutin on skin aging. Int J Mol Med 38: 357–363. https://doi.org/10.3892/ijmm.2016.2604
Sthijns M, Schiffers PM, Janssen GM, Lemmens K, Ides B, Vangrieken P, Bouwman FG, Mariman EC, Pader I, Arnér E, Johansson K, Bast A, Haenen G (2017) Rutin protects against H2O2-triggered impaired relaxation of placental arterioles and induces Nrf2-mediated adaptation in Human Umbilical Vein Endothelial Cells exposed to oxidative stress. Bba-Gen Subjects 1861: 1177–1189. https://doi.org/10.1016/j.bbagen.2017.03.004
Liu C, Li H, Xu F, Jiang X, Ma H, Seeram NP (2021) Cannabidiol Protects Human Skin Keratinocytes from Hydrogen-Peroxide-Induced Oxidative Stress via Modulation of the Caspase-1-IL-1beta Axis. J Nat Prod 84: 1563–1572. https://doi.org/10.1021/acs.jnatprod.1c00083
Yu N, Liu S, Yi X, Zhang S, Ding Y (2015) Serum amyloid A induces interleukin-1β secretion from keratinocytes via the NACHT, LRR and PYD domains-containing protein 3 inflammasome. CLIN EXP IMMUNOL 179: 344–353. https://doi.org/10.1111/cei.12458
Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH (2010) Role of oxidative stress in various stages of psoriasis. Indian J Clin Bioche 25: 388–392. https://doi.org/10.1007/s12291-010-0043-9
Tyrrell RM (2012) Modulation of gene expression by the oxidative stress generated in human skin cells by UVA radiation and the restoration of redox homeostasis. Photoch Photobio Sci 11: 135–147. https://doi.org/10.1039/c1pp05222e
Smith G, Dawe RS, Clark C, Evans AT, Comrie MM, Wolf CR, Ferguson J, Ibbotson SH (2003) Quantitative real-time reverse transcription-polymerase chain reaction analysis of drug metabolizing and cytoprotective genes in psoriasis and regulation by ultraviolet radiation. J Invest Dermatol 121: 390–398. https://doi.org/10.1046/j.1523-1747.2003.12354.x
Yang C, Ling H, Zhang M, Yang Z, Wang X, Zeng F, Wang C, Feng J (2011) Oxidative stress mediates chemical hypoxia-induced injury and inflammation by activating NF-κb-COX-2 pathway in HaCaT cells. Mol Cells 31: 531–538. https://doi.org/10.1007/s10059-011-1025-3
Matschke V, Theiss C, Matschke J (2019) Oxidative stress: the lowest common denominator of multiple diseases. Neural Regen Res 14: 238–241. https://doi.org/10.4103/1673-5374.244780
Liu Y, Xiang D, Zhang H, Yao H, Wang Y (2020) Hypoxia-Inducible Factor-1: A Potential Target to Treat Acute Lung Injury. Oxid Med Cell Longev 2020: 8871476. https://doi.org/10.1155/2020/8871476
Zhang Y, Xu M, Hu C, Liu A, Chen J, Gu C, Zhang X, You C, Tong H, Wu M, Chen P (2019) Sargassum fusiforme Fucoidan SP2 Extends the Lifespan of Drosophila melanogaster by Upregulating the Nrf2-Mediated Antioxidant Signaling Pathway. Oxid Med Cell Longev 2019: 8918914. https://doi.org/10.1155/2019/8918914
Huang D, Yin L, Liu X, Lv B, Xie Z, Wang X, Yu B, Zhang Y (2018) Geraniin protects bone marrow derived mesenchymal stem cells against hydrogen peroxide-induced cellular oxidative stress in vitro. Int J Mol Med 41: 739–748. https://doi.org/10.3892/ijmm.2017.3276
Shi X, Shi Z, Huang H, Zhu H, Zhu H, Ju D, Zhou P (2013) PEGylated human catalase elicits potent therapeutic effects on H1N1 influenza-induced pneumonia in mice. Appl Microbiol Biot 97: 10025–10033. https://doi.org/10.1007/s00253-013-4775-3
Khan MM, Ahmad A, Ishrat T, Khuwaja G, Srivastawa P, Khan MB, Raza SS, Javed H, Vaibhav K, Khan A, Islam F (2009) Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res 1292: 123–135. https://doi.org/10.1016/j.brainres.2009.07.026
Nkpaa KW, Onyeso GI (2018) Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats. Neurosci Lett 682: 92–99. https://doi.org/10.1016/j.neulet.2018.06.023
Tseng JC, Chang YC, Huang CM, Hsu LC, Chuang TH (2021) Therapeutic Development Based on the Immunopathogenic Mechanisms of Psoriasis. Pharmaceutics 13: 1064. https://doi.org/10.3390/pharmaceutics13071064
Mantovani F, Collavin L, Del Sal G (2019) Mutant p53 as a guardian of the cancer cell. Cell Death Differ 26: 199–212. https://doi.org/10.1038/s41418-018-0246-9
Athari SS (2019) Targeting cell signaling in allergic asthma. Signal Transduction And Targeted Therapy 4: 45. https://doi.org/10.1038/s41392-019-0079-0
Jeong Y, Hoang NT, Lovejoy A, Stehr H, Newman AM, Gentles AJ, Kong W, Truong D, Martin S, Chaudhuri A, Heiser D, Zhou L, Say C, Carter JN, Hiniker SM, Loo BW, West RB Jr, Beachy P, Alizadeh AA, Diehn M (2017) Role of KEAP1/NRF2 and TP53 Mutations in Lung Squamous Cell Carcinoma Development and Radiation Resistance. Cancer Discov 7: 86–101. https://doi.org/10.1158/2159-8290.CD-16-0127
ACKNOWLEDGMENTS
The authors thank Kai-Yuan Pan from Key Laboratory of Basic Pharmacology of Ministry of Education, China for his excellent technical assistance.
Funding
This study was supported by Science and Technology Fund Project of Guizhou Provincial Health Commission (gzwjkj2020-1-087), Guizhou Administration of Traditional Chinese Medicine, Ethnic Medicine Science and Technology Research Project (QZYY-2021-017), Natural Science and Technology Foundation of Guizhou Province ((2020) 4Y095, (2020) 4Y066).
Author information
Authors and Affiliations
Contributions
GPL: conducting experiments; GPL, YYH: experimental data analysis, statistical data processing, preparing graphical material, writing and editing a manuscript.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All ethical standards set forth by the Zunyi Medical University and local laws were complied with.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest, both evident and potential, that would be associated with the publication of this article.
Rights and permissions
About this article
Cite this article
Lang, GP., Han, YY. Rutin Ameliorates H2O2-Induced Oxidative Stress Injury in HaCaT Cells via the Nrf2-Regulated Pathway. J Evol Biochem Phys 58, 1389–1400 (2022). https://doi.org/10.1134/S0022093022050106
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022050106